Your What is the volume of 1 mole of copper images are available. What is the volume of 1 mole of copper are a topic that is being searched for and liked by netizens now. You can Find and Download the What is the volume of 1 mole of copper files here. Find and Download all free photos.
If you’re looking for what is the volume of 1 mole of copper images information linked to the what is the volume of 1 mole of copper keyword, you have come to the ideal site. Our site frequently provides you with suggestions for seeing the maximum quality video and image content, please kindly search and find more informative video content and graphics that fit your interests.
What Is The Volume Of 1 Mole Of Copper. How many moles of carbon atoms are there in 0500 mole of. 425 x 1026 atoms of barium 1 mol 706 mol 602 x 1023 atoms 3. A 0500 mole B 100 mole C 300 moles D 602 moles E 400 moles. 10 g gold 1 mole 602 x 1023 atoms 306 x 1022 atoms 1970g 1 mole PRACTICE PROBLEMS.
 The Mole Ppt Download From slideplayer.com
The Mole Ppt Download From slideplayer.com
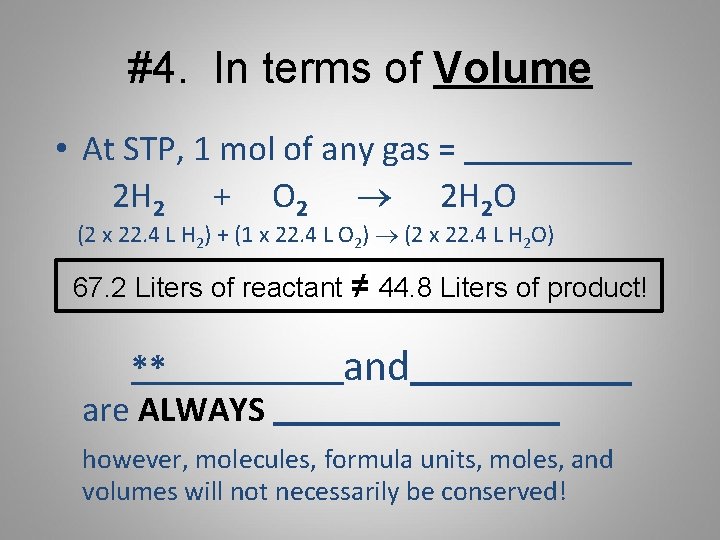
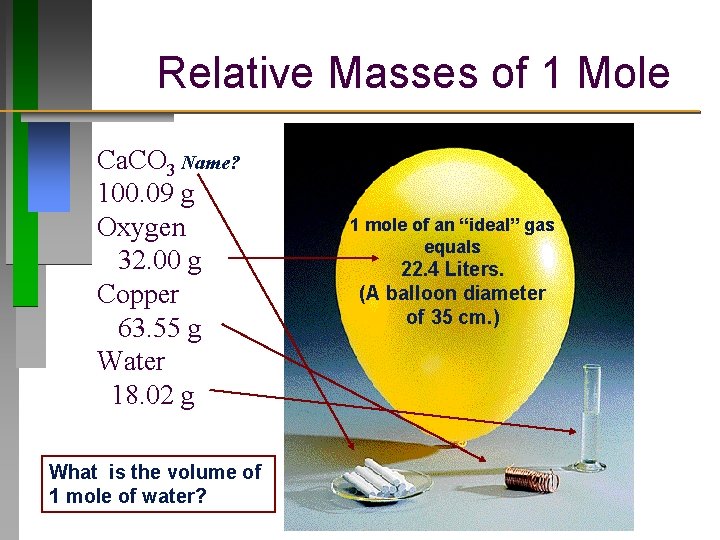
1M CuSO4 copper sulphate 1mole of CuSO4 in 1 L that is 1000 mL of solution 01 mole of CuSO4 in 100ml of solution. One mole of copper has a mass. What is the volume V of a sample of 280 mol of copper. 1 mole is equal to 1 moles Copper or 63546 grams. A 100 mL sample of this stock solution is added to500 mL. In order to find the mass when giving the amount of moles just multiply the moles by the molar mass.
How many moles of.
If 15 mol of copper sulfate is dissolved to make a solution with a volume. Copper has 2 known isotopes. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. Type in your own numbers in the form to convert the units. A 100 mL sample of this stock solution is added to500 mL. How many moles are equal to 625g of copper.
 Source: slidetodoc.com
Source: slidetodoc.com
The atomic mass of copper Cu is 635 gmol and the density of copper is 892 103 kgm3. How many grams are equal to 011 mole of copperI chromate Cu2CrO4. Therefore there is 01 mole of CuSO4 in 100 mL of 1M solution. Copper weighs 894. The atomic mass of copper Cu is 635 gmol and the density of copper is 892 103 kgm3.
 Source: pinterest.com
Source: pinterest.com
127 x 1023 molecules. Calculate the volume of gas liberated at the anode at STP during the electrolysis of a CuSO4 solution by a current of 1 A passed for 16 minutes and 5 seconds. Express your answer in cubic centimeters to 3 significant figures. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. The mass of the CuSO4 x 5H2O sample is 1664 g.
 Source: pinterest.com
Source: pinterest.com
The mass of the CuSO4 x 5H2O sample is 1664 g. A 0500 mole B 100 mole C 300 moles D 602 moles E 400 moles. 1 mole is equal to 1 moles Copper or 63546 grams. Answer 1 of 3. CAS Registry Number CAS RN.
 Source: pinterest.com
Source: pinterest.com
How many moles of. Express your answer in cubic centimeters to 3 significant figures. A solution is prepared by dissolving 250 g of ammonium sulfate in enough water to make 1000 mL of stock solution. How many moles of carbon atoms are there in 0500 mole of. Therefore there is 01 mole of CuSO4 in 100 mL of 1M solution.
 Source: slideplayer.com
Source: slideplayer.com
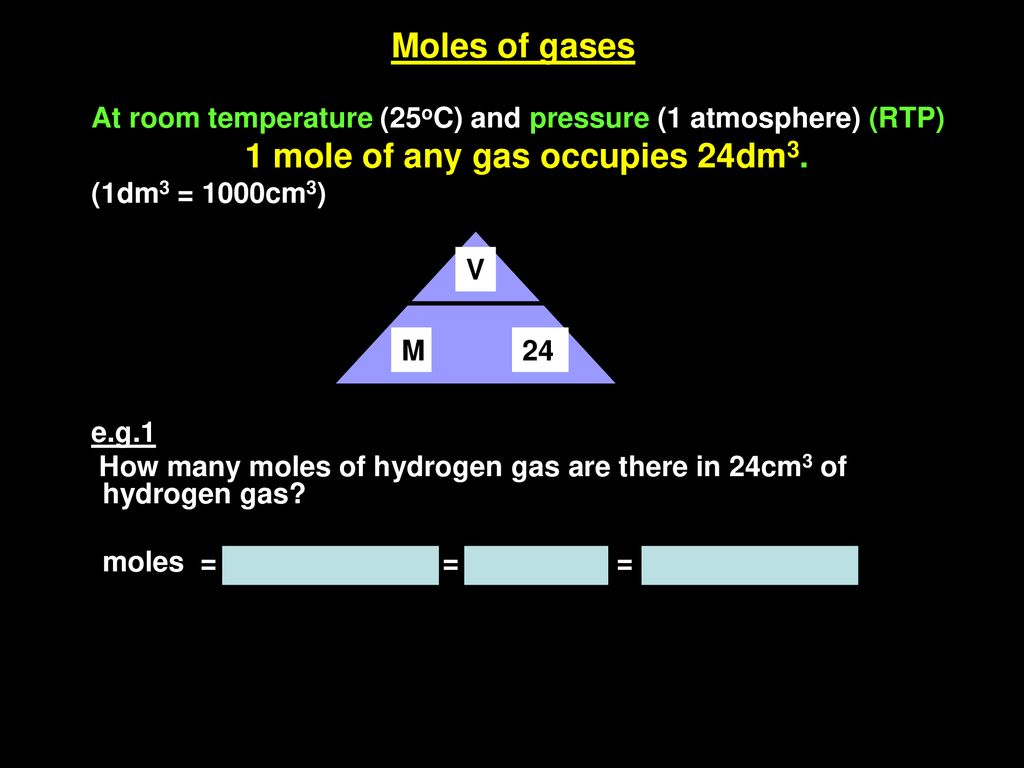
Molar mass of copper 635 grams number of moles mass molar mass 23414 mass 635 mass 23414 x 635 1486789 grams Then we get the volume. What volume would 225 moles of Ne gas occupy at STP. In order to find the mass when giving the amount of moles just multiply the moles by the molar mass. Use this page to learn how to convert between moles Copper and gram. Answer 1 of 3.
 Source: pinterest.com
Source: pinterest.com
Express your answer in cubic centimeters to 3. How many moles of barium are in a sample containing 425 x 1026 atoms of barium. What volume would 225 moles of Ne gas occupy at STP. Molar mass of copper 635 grams number of moles mass molar mass 23414 mass 635 mass 23414 x 635 1486789 grams Then we get the volume. 1M CuSO4 copper sulphate 1mole of CuSO4 in 1 L that is 1000 mL of solution 01 mole of CuSO4 in 100ml of solution.
 Source: slideplayer.com
Source: slideplayer.com
The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. How many moles of carbon atoms are there in 0500 mole of. Molar mass of copper 635 grams number of moles mass molar mass 23414 mass 635 mass 23414 x 635 1486789 grams Then we get the volume. The acid and alkali react in a 11 ratio. The atomic mass of copper Cu is 635 gmol and the density of copper is 892 103 kgm3.
 Source: pinterest.com
Source: pinterest.com
Sulphate ions 01 mole 01 x 6022 x 1023 6022 x 1022 ions. 127 x 1023 molecules. CAS Registry Number CAS RN. 625g of copper 1 mol 977 mol Cu 64 g Cu 2. The mass of the CuSO4 x 5H2O sample is 1664 g.
 Source: pinterest.com
Source: pinterest.com
1 mole is equal to 1 moles Copper or 63546 grams. 6356Cu 3207S 41599O4 1596gCuSO4 Now consider that MnL and input 250 in for L considering the conversion of mL to L and 01 in for M to find the number of moles necessary for the target con. To begin you must calculate the molar mass of copper ii sulfate. Therefore there is 01 mole of CuSO4 in 100 mL of 1M solution. How many moles of.
 Source: pinterest.com
Source: pinterest.com
What is the volume V of a sample of 280 mol of copper. 10 g gold 1 mole 602 x 1023 atoms 306 x 1022 atoms 1970g 1 mole PRACTICE PROBLEMS. Finding molar mass starts with units of grams per mole gmol. Copper has 2 known isotopes. Copper weighs 894.
 Source: pinterest.com
Source: pinterest.com
How many molecules are in 135 g of sulfur dioxide SO2. Express your answer in cubic centimeters to 3. 9 rows 1 000 000 000 000. 425 x 1026 atoms of barium 1 mol 706 mol 602 x 1023 atoms 3. Our table of molar volumes has over 130 values covering 97 elements.
 Source: slideplayer.com
Source: slideplayer.com
Weight to volume volume to weight price mole to volume and weight mass and molar concentration density A few materials substances compounds or elements with a name containing like or similar to Copper. A 0500 mole B 100 mole C 300 moles D 602 moles E 400 moles. What is the volume V of a sample of 280 mol of copper. Calculate the volume of gas liberated at the anode at STP during the electrolysis of a CuSO4 solution by a current of 1 A passed for 16 minutes and 5 seconds. The mass of the recovered copper is 1198 g.
 Source: pinterest.com
Source: pinterest.com
Number of moles 1411024 x 1 6022 x 1023 23414 moles From the periodic table. Use this page to learn how to convert between moles Copper and gram. 127 x 1023 molecules. The moles of a gas are directly proportional to the gas constant R. 1M CuSO4 copper sulphate 1mole of CuSO4 in 1 L that is 1000 mL of solution 01 mole of CuSO4 in 100ml of solution.
 Source: slidetodoc.com
Source: slidetodoc.com
The SI base unit for amount of substance is the mole. 1 10 -6. The mass of the recovered copper is 1198 g. Physical science 8th grade. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance.
 Source: slideplayer.com
Source: slideplayer.com
Sulphate ions 01 mole 01 x 6022 x 1023 6022 x 1022 ions. How many moles of barium are in a sample containing 425 x 1026 atoms of barium. Answer 1 of 3. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. The atomic mass of copper Cu is 635 gmol and the density of copper is 892103kgm3.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title what is the volume of 1 mole of copper by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






