Your What is the volume of 1 mole of carbon dioxide at stp images are ready in this website. What is the volume of 1 mole of carbon dioxide at stp are a topic that is being searched for and liked by netizens now. You can Download the What is the volume of 1 mole of carbon dioxide at stp files here. Find and Download all royalty-free photos.
If you’re looking for what is the volume of 1 mole of carbon dioxide at stp pictures information related to the what is the volume of 1 mole of carbon dioxide at stp keyword, you have visit the right blog. Our site frequently provides you with suggestions for seeking the maximum quality video and image content, please kindly surf and find more enlightening video articles and images that fit your interests.
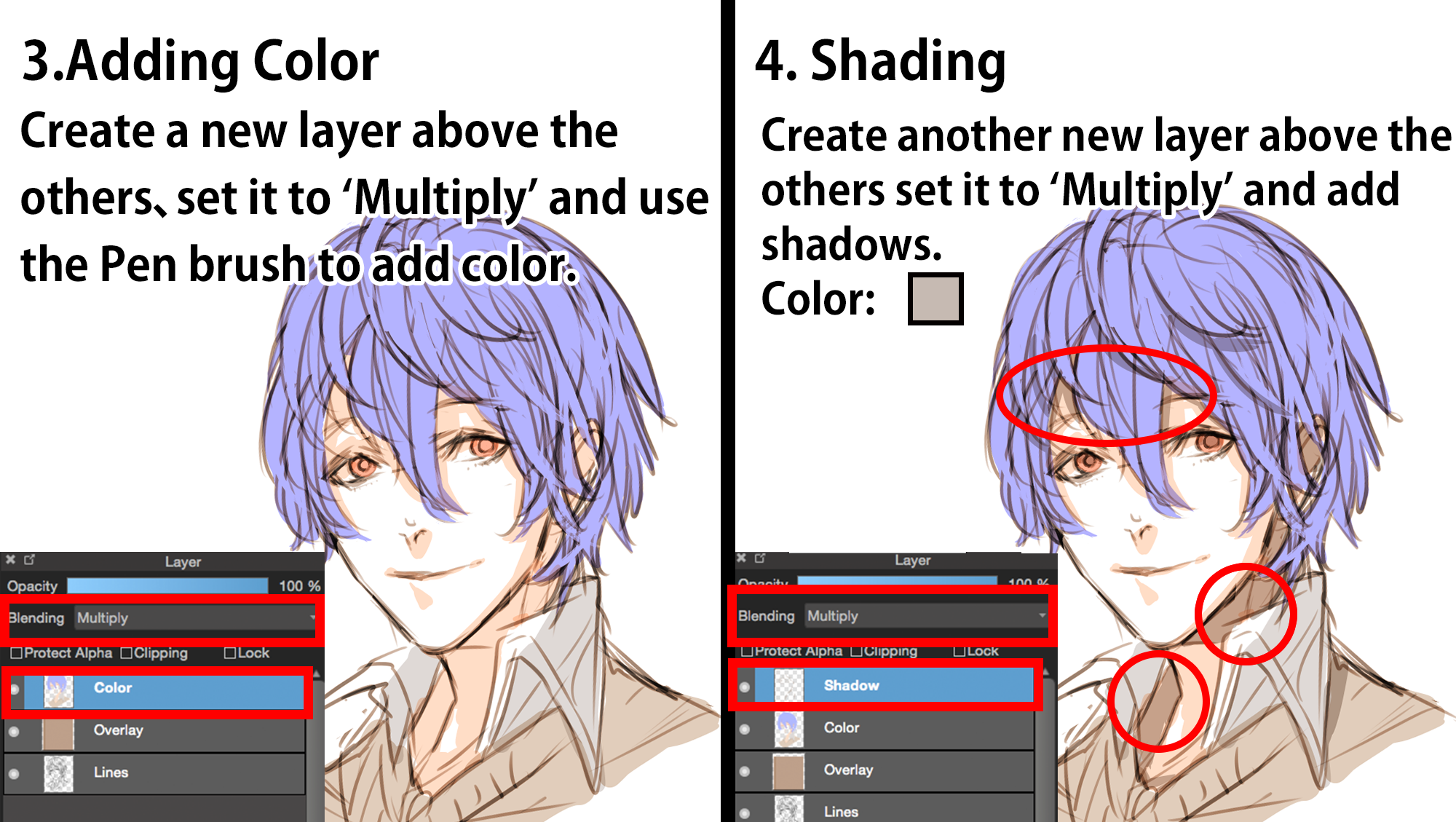
What Is The Volume Of 1 Mole Of Carbon Dioxide At Stp. Note that I chose the R constant appropriately. The information that carbon dioxide CO2 is at STP standard temperature and pressure tells us that one mole of the gas occupies 224 liters. 1 mole of gas at STP 224 liters of gas. Where V m is the volume of the substance.
 What Volume Of Co2 Is Obtained At Stp By Heating 4g Of Caco3 From toppr.com
What Volume Of Co2 Is Obtained At Stp By Heating 4g Of Caco3 From toppr.com
Double bonds in the CO 2 molecules e. Any gas occupies 224 L at standard temperature and pressure 0C and 1 atm. Then what is the molar volume of carbon dioxide. 1 mole of any gas at STP 224 Liters 5 g CO_2g 5 g44gmole 0114 mole CO_2g Volume of 0114 mole CO_2g 0114 mole224 Lmole 255 Liters CO_2 g at STP. In this manner what is the molar volume of carbon dioxide at STP. At STP one mole 602 1023 representative particles of any gas occupies a volume of 224 L Figure below.
As we know that at STP 1 mole of substance always contains 224 L volume of substance.
Standard Temperature 0 o C or 273 K. ANswer30 option D because at STP 1 mole of ideal gas occupies 224 litres View the full answer. The SI unit of molar volume is cubic meters per mole m 3 mol. You know the volume of 10 mol of carbon dioxide at STP. And we then use the Ideal Gas Equation to get our volume. Note that I chose the R constant appropriately.
 Source: toppr.com
Source: toppr.com
You know the volume of 10 mol of carbon dioxide at STP. The SI unit of molar volume is cubic meters per mole m 3 mol. At STP one mole 602 1023 representative particles of any gas occupies a volume of 224 L Figure below. Weight to volume volume to weight price mole to volume and weight mass and molar concentration. The volume of one mole of CO2 produced is 24 dm3 at room temperature and pressure.

Standard Temperature and Pressure. Standard Pressure 1 atm or 760 mm of mercury. This means that one mole of any gas occupies the same volume at STP which is 224 dm 3. Standard Temperature 0 o C or 273 K. ANswer30 option D because at STP 1 mole of ideal gas occupies 224 litres View the full answer.
 Source: youtube.com
Source: youtube.com
The molar mass of carbon dioxide is 4401 grams. Where V m is the volume of the substance. Carbon dioxide is a colorless odorless incombustible gas resulting from the oxidation of carbon. 1 mol occupies 22400 cm3. Therefore 0224L or 224ml of CO2 will be formed.
 Source: clutchprep.com
Source: clutchprep.com
The molar volume of any gas is 224 dm 3 mol-1 at STP or 24 dm 3 mol-1 at room conditions. We can identify condition 1 as the volume temperature and pressure of the CO2 sample at laboratory conditions and condition 2 as STP. Where V m is the volume of the substance. Note that I chose the R constant appropriately. You know the volume of 10 mol of carbon dioxide at STP.
 Source: chegg.com
Source: chegg.com
The molar mass of carbon dioxide is 4401 grams. 4L volume. We can identify condition 1 as the volume temperature and pressure of the CO2 sample at laboratory conditions and condition 2 as STP. Click to see full answer. Note that I chose the R constant appropriately.
 Source: youtube.com
Source: youtube.com
The volume of one mole of CO2 produced is 24 dm3 at room temperature and pressure. It is the same for all gases. Where V m is the volume of the substance. The SI unit of molar volume is cubic meters per mole m 3 mol. 300cm3 at STP of carbon dioxide.
 Source: pinterest.com
Source: pinterest.com
1 mole of gas at STP 224 liters of gas. It stands for standard temperature and pressure. Kinetic energy of the CO 2 molecules c. V 2 30 L. As we know that at STP 1 mole of substance always contains 224 L volume of substance.
 Source: toppr.com
Source: toppr.com
Furthermore What is carbon dioxide at STP Explanation. The information that carbon dioxide CO2 is at STP standard temperature and pressure tells us that one mole of the gas occupies 224 liters. Standard Temperature and Pressure. Where V m is the volume of the substance. Therefore 0224L or 224ml of CO2 will be formed.
 Source: toppr.com
Source: toppr.com
1 10 -6. Intermolecular attractions between the CO 2. It is the same for all gases. However because that is such a large volume other units are usually used. 300cm3 at STP of carbon dioxide.
 Source: pinterest.com
Source: pinterest.com
Thus the volume occupied by 01 moles of CO 2. The molar volume of any gas is 224 dm 3 mol-1 at STP or 24 dm 3 mol-1 at room conditions. Weight to volume volume to weight price mole to volume and weight mass and molar concentration. The SI unit of molar volume is cubic meters per mole m 3 mol. Standard molar volume of a gas is the volume occupied by 1 mole of any gas at 273 K and 1 atm pressure STP.
 Source: toppr.com
Source: toppr.com
The mass of the ideal gas can be calculated using its molecular weight. STP refers to standard temperature of 0C and pressure of 1 atmosphere. Furthermore What is carbon dioxide at STP Explanation. The molar volume of gas is 24 dm 3 at RTP room temperature and pressure. The information that carbon dioxide CO2 is at STP standard temperature and pressure tells us that one mole of the gas occupies 224 liters.

ANswer30 option D because at STP 1 mole of ideal gas occupies 224 litres View the full answer. At standard temperature and pressure the density of carbon dioxide is around 198 kgm 3 about 153 times that of air. The molar mass of CO2 is 1201 gmol216 gmol4401 gmol. 1 10 -6. 1 mole of gas at STP 224 liters of gas.
 Source: toppr.com
Source: toppr.com
We can identify condition 1 as the volume temperature and pressure of the CO2 sample at laboratory conditions and condition 2 as STP. The volume of one mole of CO2 produced is 24 dm3 at room temperature and pressure. You need to find the mass of 30 L of carbon dioxide gas CO 2g. This means that one mole of any gas occupies the same volume at STP which is 224 dm 3. 4L volume.
 Source: youtube.com
Source: youtube.com
At standard temperature and pressure the density of carbon dioxide is around 198 kgm 3 about 153 times that of air. Standard molar volume of a gas is the volume occupied by 1 mole of any gas at 273 K and 1 atm pressure STP. 1 mole of gas at STP 224 liters of gas. 1 mole of a gas occupies 224L at STP. CAS Registry Number CAS RN.
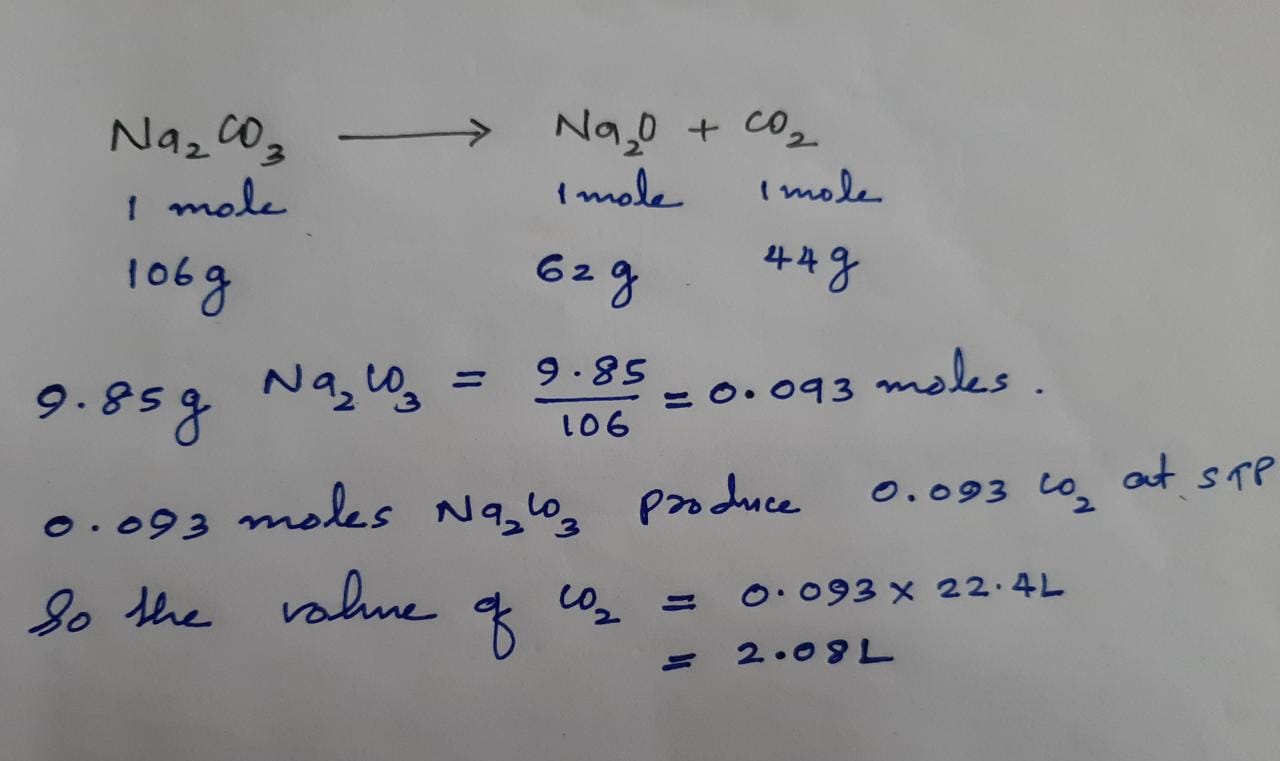 Source: digital.aakash.ac.in
Source: digital.aakash.ac.in
Then what is the molar volume of carbon dioxide. Alternatively sometimes the supplementary material in an exam gives the molar volume at STP whatever standard they have used. Intermolecular attractions between the CO 2. What mass of carbon dioxide is present in 30 L. Where V m is the volume of the substance.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title what is the volume of 1 mole of carbon dioxide at stp by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.