Your What is the solubility expression for baso4 images are available in this site. What is the solubility expression for baso4 are a topic that is being searched for and liked by netizens today. You can Download the What is the solubility expression for baso4 files here. Find and Download all free vectors.
If you’re searching for what is the solubility expression for baso4 images information connected with to the what is the solubility expression for baso4 keyword, you have visit the ideal blog. Our website always provides you with suggestions for refferencing the maximum quality video and image content, please kindly surf and find more informative video content and graphics that fit your interests.
What Is The Solubility Expression For Baso4. Given that solubility product of BaSO_4 is 1 xx 10-10 will be precipiate from when Equal volumes of 2 xx 10-3M BaCl_2 solution and 2 xx 10. However it is important to appreciate that the ionic strength of solution 1 is large enough to have an impact on the solubility. Chapter 17 Problem 67QP is solved. C_2 H_2g 3 H_2Ol rightarrow 3 COg 7 H_2g If the denominator of equilibrium expression is expressed as.

Learn vocabulary terms and more with flashcards games and other study tools. The solubility of an AB 2 type salt is 23 x 10-6 M. Click hereto get an answer to your question The solubility product Ksp of BaSO4 is 15 10-9. A Write an equation that represents the solubility equilibrium b Calculate the Ba2 present in the saturated solution. Fourth substitute the equilibrium concentrations into the equilibrium expression and solve for K sp. Consider the solubility of BaSo.
Note Na2SO4 is a common ion the sulfate ion is the common ion.
The rate constant is dependent on the reactant concentrations. What does that do. In a series of stepwise reactions the rate. Solution for The solubility product Ksp for BaSO4 is 11 1010. The principle difference is that the solution has Na2SO4 in it. Note Na2SO4 is a common ion the sulfate ion is the common ion.
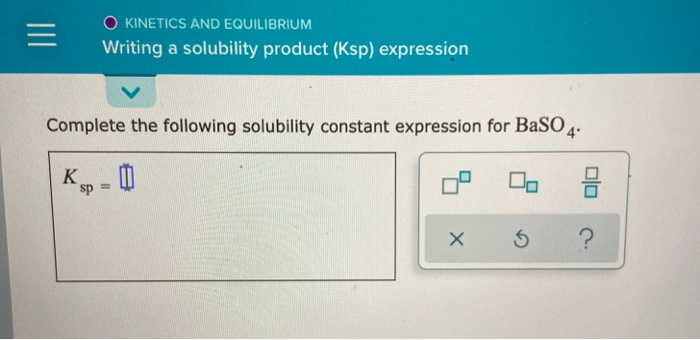 Source: chegg.com
Source: chegg.com
Calculate the solubility of barium sulphate in pure water and in 01M BaCl2. 510-9 it is highly likely that the solubility of the compound in water is very small. Click hereto get an answer to your question The solubility product Ksp of BaSO4 is 15 10-9. Since in a heterogeneous equilibrium the concentration of solid BaSO4 is a constant. The solubility product Ksp for BaSO4 is 11 10-10.
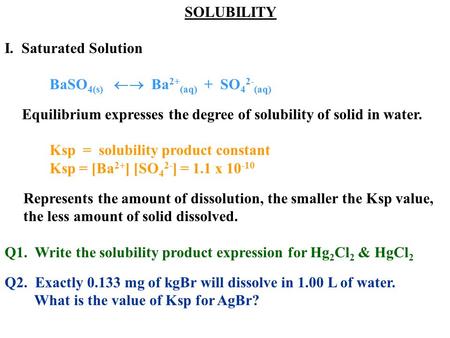 Source: slideplayer.com
Source: slideplayer.com
Apr 7 2013. Thus solubility of BaSO4 will be 1225 10 –5 moll. The Ksp for BaS04 which is barium sulfate is 11 x 10-10. Solubility product In general solubility product Ksp is the mathematical product of its dissolved ion concentrations raised to the power of their stoichiometric coefficients. The SO4 is a common ion as it appears in the solubility equilibrium.
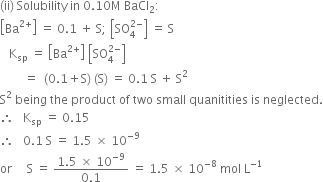 Source: zigya.com
Source: zigya.com
Let us set up the equilibrium for the above reaction. Sulfate comes from two sources. Click hereto get an answer to your question The solubility product Ksp of BaSO4 is 15 10-9. C_2H_2 H_2O2 what are the values of m and n. Solution for The solubility product Ksp for BaSO4 is 11 1010.

C_2 H_2g 3 H_2Ol rightarrow 3 COg 7 H_2g If the denominator of equilibrium expression is expressed as. BaSO4 is a slightly soluble salt. Write the equation and the. Learn vocabulary terms and more with flashcards games and other study tools. Solution for The solubility product Ksp for BaSO4 is 11 1010.
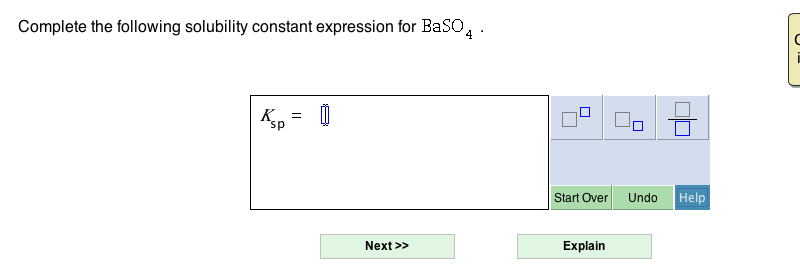 Source: chegg.com
Source: chegg.com
The solubility product Ksp for BaSO4 is 11 10-10. Consider the solubility of BaSo. BaSO4 is known as barium sulfate. Click hereto get an answer to your question The solubility product Ksp of BaSO4 is 15 10-9. C_2 H_2g 3 H_2Ol rightarrow 3 COg 7 H_2g If the denominator of equilibrium expression is expressed as.
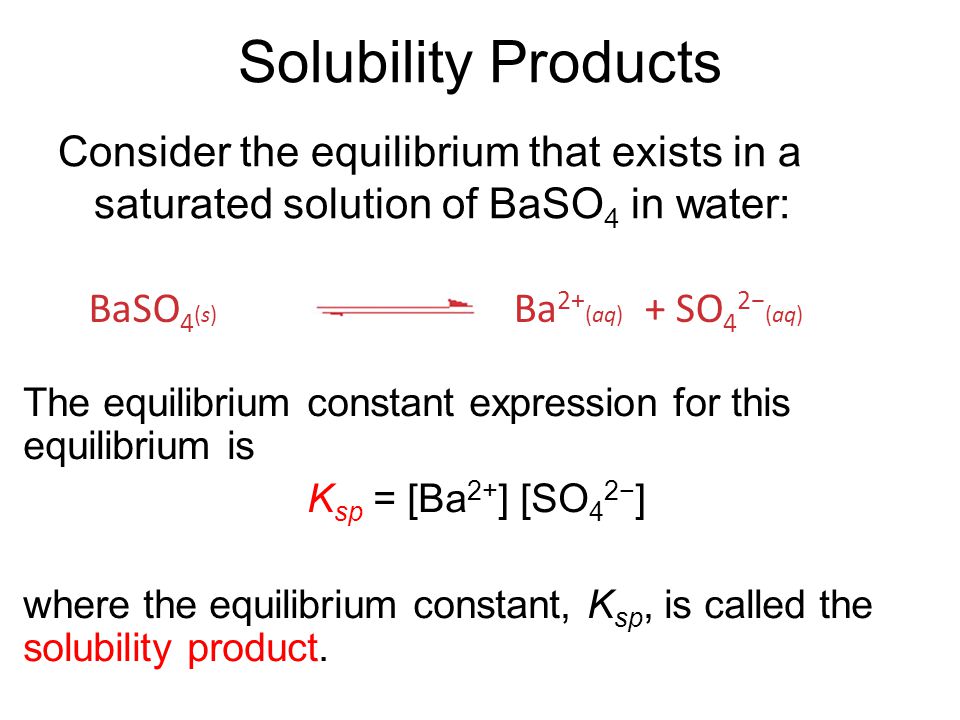 Source: slideplayer.com
Source: slideplayer.com
55 1011 mol. BaSO4 is a slightly soluble salt. Write the equation and the. Click hereto get an answer to your question The solubility product Ksp of BaSO4 is 15 10-9. The rates of most chemical reactions change with time.

Consider the solubility of BaSo. A saturated solution of BaSO 4 is given to patients needing digestive tract x-rays. The rate constant is dependent on the reactant concentrations. Since in a heterogeneous equilibrium the concentration of solid BaSO4 is a constant. The rates of most chemical reactions change with time.
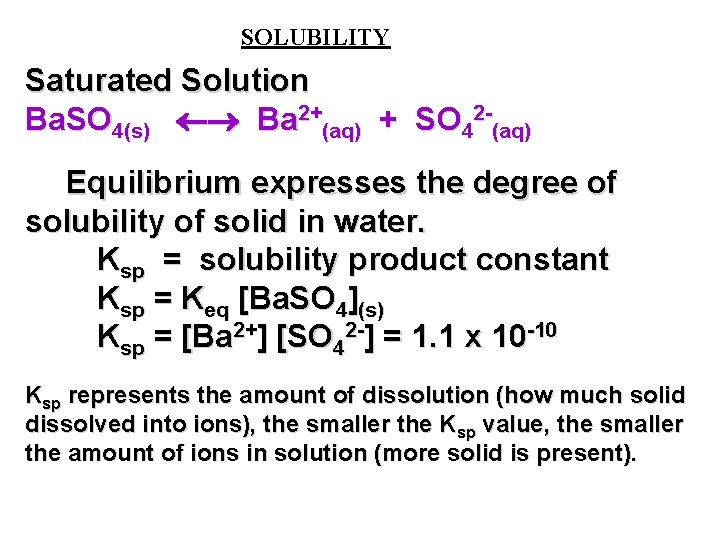 Source: slidetodoc.com
Source: slidetodoc.com
CuIO 3 2 D. The solubility product constant expression then becomes as follows. How can I calculate the molar solubility of BaSO4 given a Ksp of 1071010. Calculate the solubility of BaSO4 in 0100 M Na2SO4. Sulfate comes from two sources.
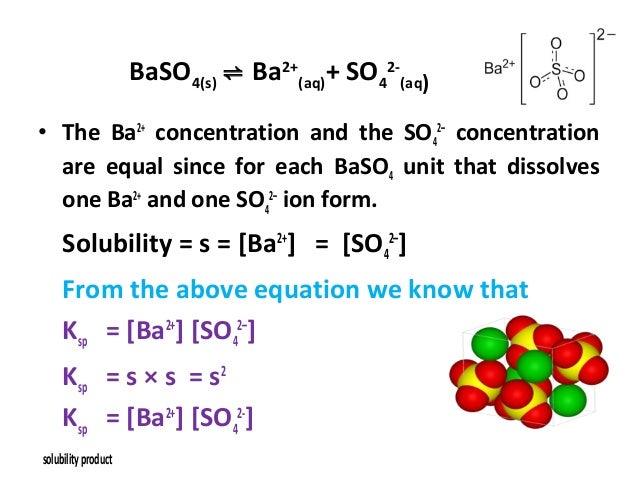 Source: pt.slideshare.net
Source: pt.slideshare.net
In a series of stepwise reactions the rate. In a series of stepwise reactions the rate. BaSO4 is known as barium sulfate. C_2H_2 H_2O2 what are the values of m and n. If BaNO_3 is added to the solution the solubility fo the barium sulfate.
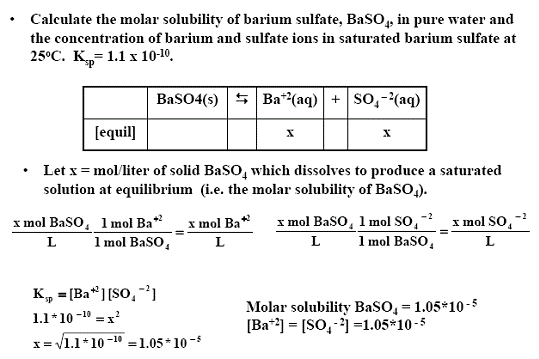 Source: chem.fsu.edu
Source: chem.fsu.edu
It is the product of the solubility of the ions in moles per liter. How can I calculate the molar solubility of BaSO4 given a Ksp of 1071010. A saturated solution of BaSO 4 is given to patients needing digestive tract x-rays. The solubility product constant expression then becomes as follows. Solution for The solubility product Ksp for BaSO4 is 11 1010.
 Source: brainly.in
Source: brainly.in
It is the product of the solubility of the ions in moles per liter. Basically when BaSO4 dissolves in water it dissociates into the ions Ba2 and SO4-2. Calculate the solubility of barium sulphate in pure water and in 01M BaCl2. Chapter 17 Problem 67QP is solved. So even for this solution it is expected that the solubility will be significantly larger than the 50 mM predicted above.
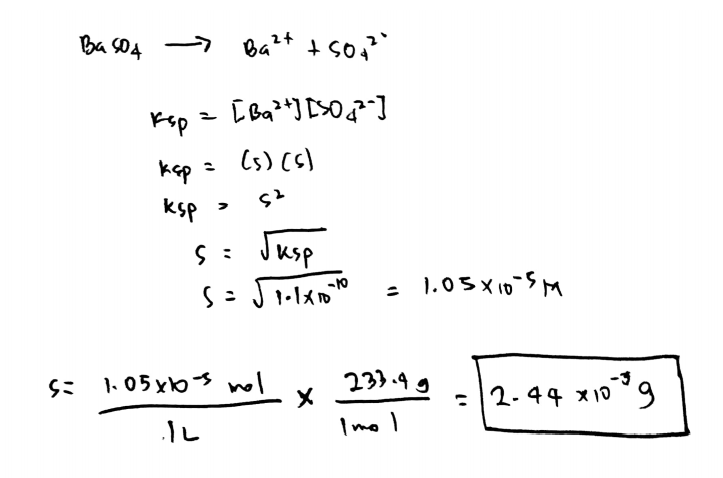 Source: clutchprep.com
Source: clutchprep.com
However it is important to appreciate that the ionic strength of solution 1 is large enough to have an impact on the solubility. Calculate the solubility of barium sulphate in pure water and in 01M BaCl2. C_2 H_2g 3 H_2Ol rightarrow 3 COg 7 H_2g If the denominator of equilibrium expression is expressed as. The salt is A. Ksp Ba2 SO4-2 You already know that the Ksp for BaSO4 is 11x10-10.
 Source: slideplayer.com
Source: slideplayer.com
If solubility product is 151010then for given reaction relation between solubility product and solubility is sproduct4S3 where S is solubility in mollitthe above relation comes from the eqation xx yy Sxyksp. Basically when BaSO4 dissolves in water it dissociates into the ions Ba2 and SO4-2. A Write an equation that represents the solubility equilibrium b Calculate the Ba2 present in the saturated solution. Note the presence of 0100 M Na2SO4. It is found elsewhere however that its solubility increases as the temperature of the water decreases.
 Source: brainly.in
Source: brainly.in
Apr 7 2013. BaSO4 s Ba2aq SO4-2 aq The equilibrium expression for this reaction is. Ksp Ba2 SO4-2 You already know that the Ksp for BaSO4 is 11x10-10. Calculate the solubility of barium sulphate in pure water and in 01M BaCl2. The SO4 is a common ion as it appears in the solubility equilibrium.
 Source: youtube.com
Source: youtube.com
CuIO 3 2 D. PH of water also plays an important role in the solubility of CaCO3. Discuss about what quantifiable and non- quantifiable factors you are going to include in your business case for an rfid system. BaSO4 s Ba2aq SO4-2 aq The equilibrium expression for this reaction is. Click to see full answer.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title what is the solubility expression for baso4 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






