Your What is the molar solubility of baso4 images are available. What is the molar solubility of baso4 are a topic that is being searched for and liked by netizens now. You can Get the What is the molar solubility of baso4 files here. Find and Download all royalty-free vectors.
If you’re searching for what is the molar solubility of baso4 images information connected with to the what is the molar solubility of baso4 topic, you have come to the right site. Our site always gives you suggestions for seeing the maximum quality video and picture content, please kindly search and locate more enlightening video articles and graphics that match your interests.
What Is The Molar Solubility Of Baso4. BaSO4 must dissolve to produce one mole of Ba2 and one mole of SO4-2 in solution. That means that the molar barium ion concentration is. Toxicity data indicates that Ba2 is toxic at levels between 1 and 15 g ingested. Given molar mass of BaSO4 233 g mol-1.
 The Solubility Of Baso4 In Water Is 2 42 X 10 3 Gl 1 At 298 K The Value Of Its Solubility Product Ksp Will Be Given Molar Mass Of Baso4 233 G Mol 1 From toppr.com
The Solubility Of Baso4 In Water Is 2 42 X 10 3 Gl 1 At 298 K The Value Of Its Solubility Product Ksp Will Be Given Molar Mass Of Baso4 233 G Mol 1 From toppr.com
What happens to the solubility of a slightly soluble compound when an ion common to the compound is added to the saturated solution. BaSO4s Ba2aq SO42aq BaSO4 Ba2. Barium sulfate is a metal sulfate with formula BaO4S. What does that do. Calculate the solubility of barium sulphate in pure water and in 01M BaCl2. The value of its solubility product Ksp will be.
BaSO4 must dissolve to produce one mole of Ba2 and one mole of SO4-2 in solution.
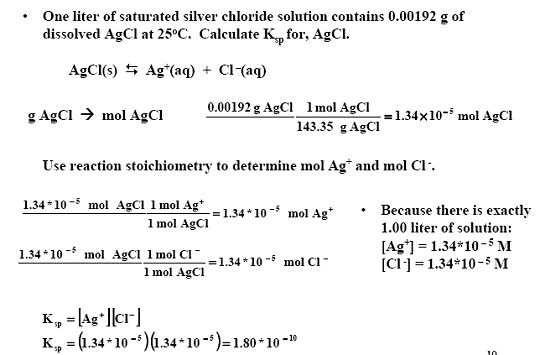
The Ksp for BaSO4 is 1110-10 at 25 C. 1 mol sulfuric acid is 98 grams of sulfuric acid. Calculate the solubility of barium sulfate in pure water in a moles per liter and b grams per liter. Sulfate comes from two sources. Virtually insoluble in water at room temperature it is mostly used as a component in oil well drilling fluid it occurs naturally as the mineral barite. Estimate the solubility of Ag 2 CrO 4 in pure water if the solubility product constant for silver chromate is 11 x 10-12.
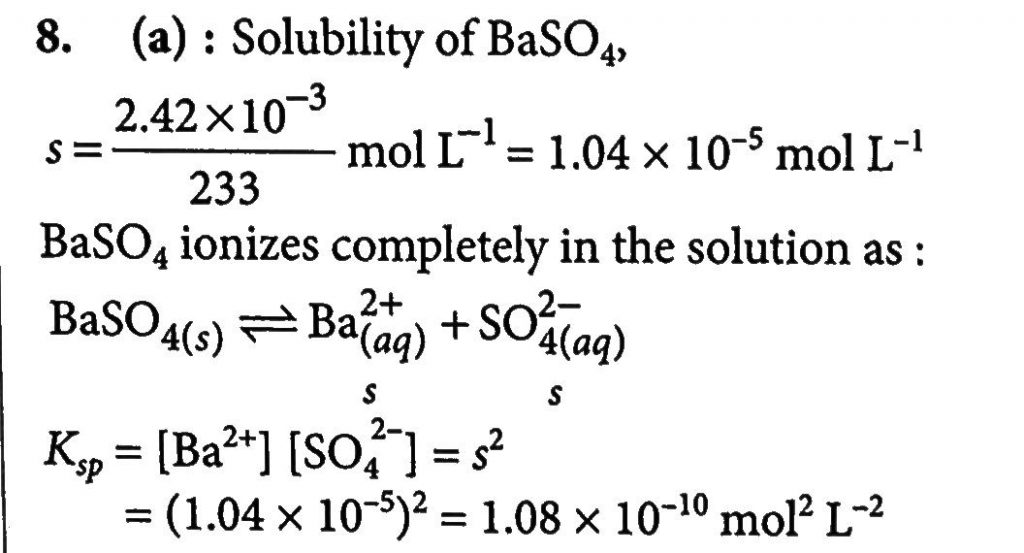 Source: sahay.guru
Source: sahay.guru
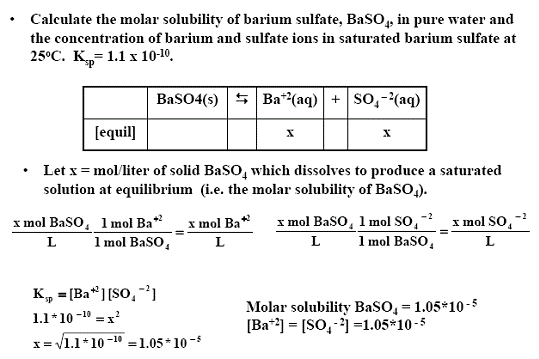
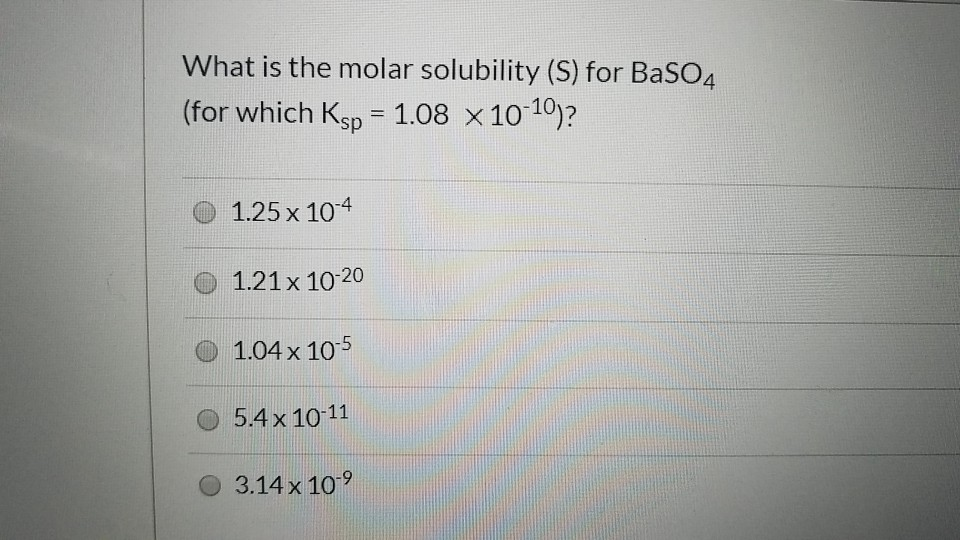
108 x 10-12 mol 2 L-2. The molar solubility S of barium sulfate BaSO4 is 105 x 10-5 M. What does that do. Toxicity data indicates that Ba2 is toxic at levels between 1 and 15 g ingested. The molar solubility s of BaSO 4 in pure water 11 10-5 M.
 Source: chem.fsu.edu
Source: chem.fsu.edu
Note Na2SO4 is a common ion the sulfate ion is the common ion. Sulfate comes from two sources. It is a barium salt and a metal sulfate. The value of its solubility product K sp will be Given the molar mass of BaSO 4 233 g mol 1 108 x 10-10 mol 2 L-2. SO42- The concentration of SO42 ions can be decreased by the addition of A BaSO4s B BaCl2s C Na2SO4s D NaNO3s.
 Source: chegg.com
Source: chegg.com
SO42- The concentration of SO42 ions can be decreased by the addition of A BaSO4s B BaCl2s C Na2SO4s D NaNO3s. In the saturated solution molar concentrations of barium and sulfate ions are the same and their product is 1110-10. The solubility of BaSO 4 in water is 242 10 3 gL 1 at 298 K. The molar solubility of BaSO4 also equals 39x10-5molL because one mole of. Virtually insoluble in water at room temperature it is mostly used as a component in oil well drilling fluid it occurs naturally as the mineral barite.
 Source: chem.fsu.edu
Source: chem.fsu.edu
The solubility of BaSO 4 in water is 242 10 3 gL 1 at 298 K. Molar solubility 967103 M Calculate the pH of the solution made by adding 050 mol of HOBr and 030 mol of KOBr to 100 L of water. Given molar mass of BaSO4 233 g mol-1. The Ksp for BaSO4 is 1110-10 at 25 C. Estimate the solubility of Ag 2 CrO 4 in pure water if the solubility product constant for silver chromate is 11 x 10-12.
 Source: toppr.com
Source: toppr.com
What happens to the solubility of a slightly soluble compound when an ion common to the compound is added to the saturated solution. 108 x 10-14 mol 2 L-2. What happens to the solubility of a slightly soluble compound when an ion common to the compound is added to the saturated solution. BaSO4 is a slightly soluble salt. The molar solubility of BaSO4 also equals 39x10-5molL because one mole of.
 Source: toppr.com
Source: toppr.com
Chapter 17 Problem 67QP is solved. The molar solubility S of barium sulfate BaSO4 is 105 x 10-5 M. K sp 0015900318 2 161 x 10-5. -The solubility of BaSO4 is 1 x 105 molesliter. Sulfate comes from two sources.
 Source: chem.fsu.edu
Source: chem.fsu.edu
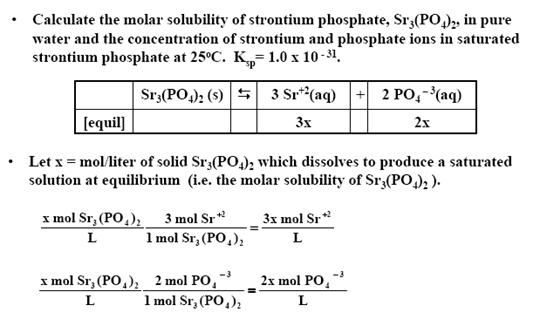
Use molar mass to change from molar solubility to solubility. Estimate the solubility of Ag 2 CrO 4 in pure water if the solubility product constant for silver chromate is 11 x 10-12. A Solubility of BaSO4 is worked similar to the CaCO3 problem. BaSO4 is a slightly soluble salt. Determination of Ksp value given the solubility One liter 10 L of saturated barium sulfate solution contains 00025 g of dissolved BaSO4.
 Source: toppr.com
Source: toppr.com
Irishluna2021 One liter 10 L of saturated solution of silver chromate at 25 oC contains 00435 g of Ag2CrO4. The value of Ka for HOBr is 20109. Sulfate comes from two sources. In the saturated solution molar concentrations of barium and sulfate ions are the same and their product is 1110-10. It has a role as a radioopaque medium.
 Source: pinterest.com
Source: pinterest.com
Added to 1 liter of saturated BaSO4 solution for practical purposes it would yield a 1 molL concentration. Click to see full answer. The molar solubility s of BaSO 4 in pure water 11 10-5 M. Added to 1 liter of saturated BaSO4 solution for practical purposes it would yield a 1 molL concentration. 108 x 10-8 mol 2 L-2.
 Source: chegg.com
Source: chegg.com
The short answer is practically all of the BaSO4 in the staurated solution. Sulfate comes from two sources. Determination of Ksp value given the solubility One liter 10 L of saturated barium sulfate solution contains 00025 g of dissolved BaSO4. Molar solubility 967103 M Calculate the pH of the solution made by adding 050 mol of HOBr and 030 mol of KOBr to 100 L of water. SO42- The concentration of SO42 ions can be decreased by the addition of A BaSO4s B BaCl2s C Na2SO4s D NaNO3s.
 Source: toppr.com
Source: toppr.com
The concentration of the ions leads to the molar solubility of the compound. The molar solubility S of barium sulfate BaSO4 is 105 x 10-5 M. In the saturated solution molar concentrations of barium and sulfate ions are the same and their product is 1110-10. The solubility data shows that the amount of barium that might dissolve in 1 L of water is only 000137 g a. The solubility product Ksp for BaSO4 is 11 10-10.
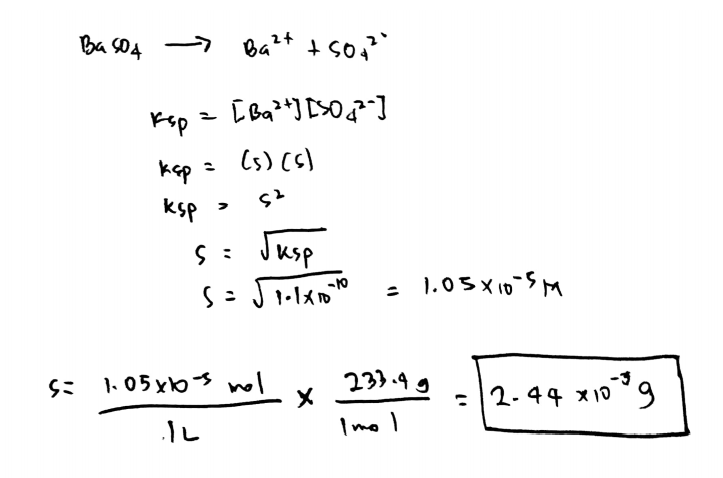 Source: clutchprep.com
Source: clutchprep.com
Note Na2SO4 is a common ion the sulfate ion is the common ion. Chapter 17 Problem 67QP is solved. What is the molar solubility of BaSO4. What happens to the solubility of a slightly soluble compound when an ion common to the compound is added to the saturated solution. Molar solubility 967103 M Calculate the pH of the solution made by adding 050 mol of HOBr and 030 mol of KOBr to 100 L of water.

The value of Ka for HOBr is 20109. 1 mol sulfuric acid is 98 grams of sulfuric acid. So the BaSO4 problem will be. Calculate the molar absorptivity of bromothymol blue at 620 nm if the measured absorbance at this wavelength is 0820 the pathlength is 100 mm. Write the equation and the.
 Source: chegg.com
Source: chegg.com
Irishluna2021 One liter 10 L of saturated solution of silver chromate at 25 oC contains 00435 g of Ag2CrO4. The solubility of BaSO 4 in water is 242 10 3 gL 1 at 298 K. Calculate the solubility product constant for BaSO4. Note Na2SO4 is a common ion the sulfate ion is the common ion. What does that do.
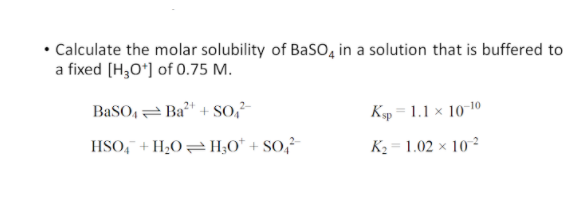 Source: chegg.com
Source: chegg.com
The Ksp for BaSO4 is 1110-10 at 25 C. Calculate the molar solubility of BaSO4 in a solution in which H3O is 050 M. BaSO4 must dissolve to produce one mole of Ba2 and one mole of SO4-2 in solution. The molar solubility s of BaSO 4 in pure water 11 10-5 M. Note Na2SO4 is a common ion the sulfate ion is the common ion.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title what is the molar solubility of baso4 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






