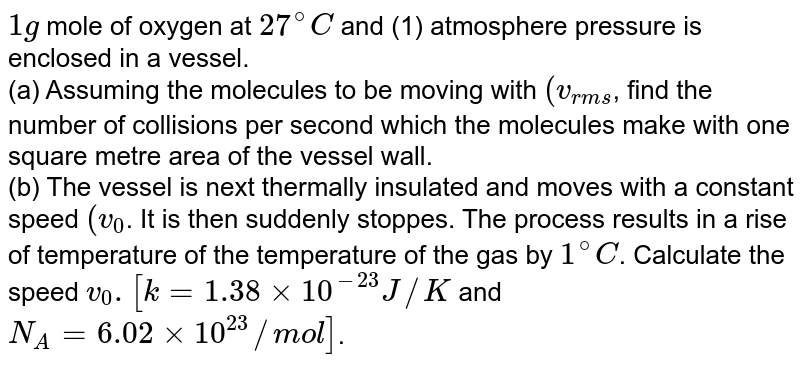
Your What is the molar mass of o2 grams per mole images are available. What is the molar mass of o2 grams per mole are a topic that is being searched for and liked by netizens now. You can Find and Download the What is the molar mass of o2 grams per mole files here. Find and Download all royalty-free images.
If you’re searching for what is the molar mass of o2 grams per mole pictures information related to the what is the molar mass of o2 grams per mole interest, you have visit the right blog. Our website always gives you hints for downloading the highest quality video and image content, please kindly surf and find more enlightening video articles and graphics that match your interests.
What Is The Molar Mass Of O2 Grams Per Mole. The atomic number of oxygen is 8. The molar mass of O2 will be. Therefore the mole is a unit for that physical quantity. First calculate the molar mass corresponding to the simplest formula CH 2 O then find the multiple by dividing the actual molar mass 60gmol by the molar mass of CH 2 O.
 Convert Into Moles A 12 G Of Oxygen Gas B 20 G Of Water C 22 G Of Carbon Dioxide Atomic Youtube From youtube.com
Convert Into Moles A 12 G Of Oxygen Gas B 20 G Of Water C 22 G Of Carbon Dioxide Atomic Youtube From youtube.com
1 mole elemental oxygen16 grams O1 mole 16 grams. And one mole of oxygen molecules has a mass of 32g so three moles would have a mass of 96g. The molar mass of O2 is 320 gmolwhat massin gramsof O2 is required to react completely with 400 mol of Mg - 10401707. MM CH 2 O 1201 gmol 21008 gmol 1600 gmol. Then convert from moles to grams. There is a unique relationship between molar mass and atomic weight.
One mole of H2O 2 602214076 1023 of Hydrogen 602214076 1023 of Oxygen.
For hydrogen we multiply the molar mass of 100794 by 4. 2 X Mass of one oxygen atom 2 16 32 gram per mole. Oxygen has a molar mass approximately 1600 g mol. Molar mass of O 159994 gmol. The atomic number of oxygen is 8. 1 mole elemental oxygen16 grams O1 mole 16 grams.
 Source: youtube.com
Source: youtube.com
Oxygen is a chemical element with symbol O and atomic number 8. There is a unique relationship between molar mass and atomic weight. Molar Mass g mol 1 Total. 15999is the molar mass of one oxygen atom. Understanding what molar mass is and how it is related to doing calculations in chemistry will help us understand why knowing oxygens molar mass is important.
 Source: youtube.com
Source: youtube.com
The molar mass of O2 will be. Avogadros number of these diatomic molecules is equal to one mole and it has a total mass in grams. Molar Mass of Air. Propane has a molar mass of 44 gmol and oxygen has a molar mass of 32 gmol. The atomic number of oxygen is 8.
 Source: youtube.com
Source: youtube.com
Since the unified atomic mass unit symbol. Since oxygen molar mass 1599 gmol does not exist as one atom so it is always O 2 32 gmol. It has a molar mass of roughly 159994. A compound with an empirical formula of CFBrO and a molar mass of 2547 grams per mole. And one mole of oxygen molecules has a mass of 32g so three moles would have a mass of 96g.
 Source: youtube.com
Source: youtube.com
M 6 l 998 kgm³ 0006 m³ 998 kgm³ 5988 kg. Avogadros number of these diatomic molecules is equal to one mole and it has a total mass in grams. Oxygen O - Standard atomic weight molar mass. X 1600 you get the number of moles. Since oxygen molar mass 1599 gmol does not exist as one atom so it is always O 2 32 gmol.

Molar mass units Gram per mole gmol Kilogram per mole kgmol. On a mass basis we need to convert the moles of the reactants to an equivalent mass. 15999is the molar mass of one oxygen atom. Multiplying the molar mass by the number of moles would give 5 moles oxygen32 gmol 160 g oxygen and 1 mole propane44 gmol 44 g propane. The molar mass of O2 is 320 gmolwhat massin gramsof O2 is required to react completely with 400 mol of Mg - 10401707.
 Source: youtube.com
Source: youtube.com
Molar Mass g mol 1 Total. What is the molar mass of NaOH. And one mole of oxygen molecules has a mass of 32g so three moles would have a mass of 96g. 1 mole of oxygen is 602 x 1023 atoms of oxygen 1 amu 1661 x 10-24g What is the molar mass gmole of oxygenMolar mass in grams is. Avogadros number of these diatomic molecules is equal to one mole and it has a total mass in grams.
 Source: youtube.com
Source: youtube.com
O2 molar mass is 32 gram per mole. Molar Mass of Air. Multiplying the molar mass by the number of moles would give 5 moles oxygen32 gmol 160 g oxygen and 1 mole propane44 gmol 44 g propane. On a mass basis we need to convert the moles of the reactants to an equivalent mass. Oxygen O 2 021.
 Source: topperlearning.com
Source: topperlearning.com
As you already know how the grams to moles conversion work find the number of moles. And one mole of oxygen molecules has a mass of 32g so three moles would have a mass of 96g. Avogadros number of these diatomic molecules is equal to one mole and it has a total mass in grams. The molar mass of individual elements helps in calculating compounds as well. Oxygen has a molar mass approximately 1600 g mol.
 Source: youtube.com
Source: youtube.com
From Avogadros law and the definition of the mole. The molar mass of O2 is 320 gmolwhat massin gramsof O2 is required to react completely with 400 mol of Mg - 10401707. 1 mole of oxygen is 602 x 1023 atoms of oxygen 1 amu 1661 x 10-24g What is the molar mass gmole of oxygenMolar mass in grams is. Nitrogen N 2 079. Therefore the mole is a unit for that physical quantity.
 Source: youtube.com
Source: youtube.com
However you would never get moles of oxygen atoms as oxygen always pairs up into molecules of O2. 15999is the molar mass of one oxygen atom. MMR of water H 2 O 18016 u. One mole of H2O 2 602214076 1023 of Hydrogen 602214076 1023 of Oxygen. Oxygens atomic weight is 1600 amu.
 Source: chem.libretexts.org
Source: chem.libretexts.org
A compound with an empirical formula of C2OH4 and a molar mass of 88 grams per mole. Since the unified atomic mass unit symbol. A compound with an empirical formula of C4H4O and a molar mass of 136 grams per mole. Oxygen molecular weight. The molar mass of an element or molecule is the total mass in grams of all the atoms that comprise a mole of a certain molecule.
 Source: youtube.com
Source: youtube.com
Understanding what molar mass is and how it is related to doing calculations in chemistry will help us understand why knowing oxygens molar mass is important. Oxygen is a chemical element with symbol O and atomic number 8. As you already know how the grams to moles conversion work find the number of moles. Molar Mass of Air. 2 X Mass of one oxygen atom 2 16 32 gram per mole.
 Source: theeducationtraining.com
Source: theeducationtraining.com
First calculate the molar mass corresponding to the simplest formula CH 2 O then find the multiple by dividing the actual molar mass 60gmol by the molar mass of CH 2 O. Propane has a molar mass of 44 gmol and oxygen has a molar mass of 32 gmol. This equals 240214 grams per mole. Note that rounding errors may occur so always check the results. A compound with an empirical formula of C4H4O and a molar mass of 136 grams per mole.
 Source: toppr.com
Source: toppr.com
Therefore the mole is a unit for that physical quantity. Atomic weight of oxygen is 15999 so a mole of oxygen atoms ie. N 5988 g 18015 gmol 3324 mol. As you already know how the grams to moles conversion work find the number of moles. For hydrogen we multiply the molar mass of 100794 by 4.
 Source: khanacademy.org
Source: khanacademy.org
Therefore the mole is a unit for that physical quantity. MM CH 2 O 1201 gmol 21008 gmol 1600 gmol. On a mass basis we need to convert the moles of the reactants to an equivalent mass. 1 mole elemental oxygen16 grams O1 mole 16 grams. As you already know how the grams to moles conversion work find the number of moles.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title what is the molar mass of o2 grams per mole by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.