Your What is the mass of 01 mole of hcl images are available in this site. What is the mass of 01 mole of hcl are a topic that is being searched for and liked by netizens now. You can Find and Download the What is the mass of 01 mole of hcl files here. Get all royalty-free photos and vectors.
If you’re looking for what is the mass of 01 mole of hcl images information related to the what is the mass of 01 mole of hcl keyword, you have come to the right blog. Our website frequently provides you with hints for refferencing the maximum quality video and image content, please kindly surf and locate more enlightening video articles and images that fit your interests.
What Is The Mass Of 01 Mole Of Hcl. This can be done by multiplying 30 mol by the molecular weight of HCl which is 3646 gmol. Moles of HCl in. HCl molecular weight. 35cm 3 of 1moldm 3 of H 2 SO 4 was required to neutralise 265cm 3 of the unknown KOH.
 Important Questions Class 11 Chemistry Chapter 1 Basic Concepts Chemistry 1 Chemistry Basic Concepts 11th Chemistry From in.pinterest.com
Important Questions Class 11 Chemistry Chapter 1 Basic Concepts Chemistry 1 Chemistry Basic Concepts 11th Chemistry From in.pinterest.com
1 kg of water. This can be done by multiplying 30 mol by the molecular weight of HCl which is 3646 gmol. Chapter 5 Molarity can be used as a conversion factor. MgCl2 H2From the equation 1mole of Mg required 2moles of HCl Therefore 15moles of Mg will require 15 x 2 3 mo. Now we have to convert the 30 mol of HCl into grams of HCl. So the mass of water present 100 - 20 grams 80 grams.
Zn s 2 HCl aq ZnCl2 aq H2 g For the calculations in this module the molar mass of an element will be rounded to the hundredths place 001 g.
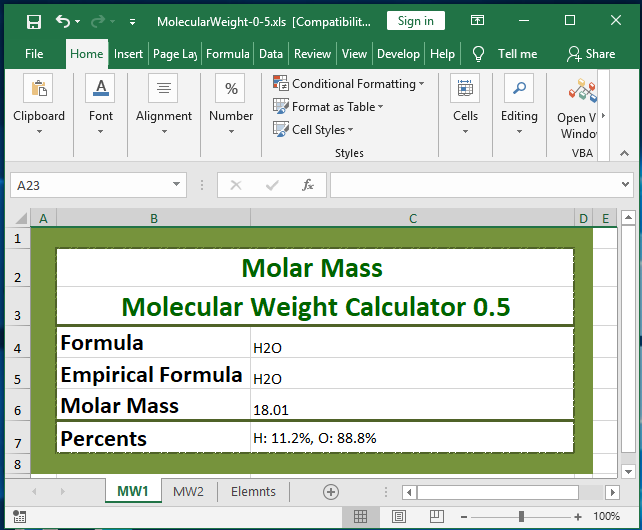
-Remember that the sum of mole fractions of all the components of mixture or a solution is always equal to 1. C 155 m 6. Hence the mole fraction of HCl is 01 and mole fraction of water is 09 in a 20 ww aqueous HCl solution. To prepare 1 L of 05 N HCl solution you have to take 05 gram equivalent of pure HCl. Of moles of HCLMass of HCL providedAtomic mass of HCL3460365948 moles no. 1 kg of water.
 Source: slidetodoc.com
Source: slidetodoc.com
MgCl2 H2From the equation 1mole of Mg required 2moles of HCl Therefore 15moles of Mg will require 15 x 2 3 mo. A 361 HCl by mass. Find the unknown concentration of KOH. Concentrated HCl is 12 M which means 12 molesL or 0012 molesmL. Of moles x Avogadro number948 x 6022 x 1023.
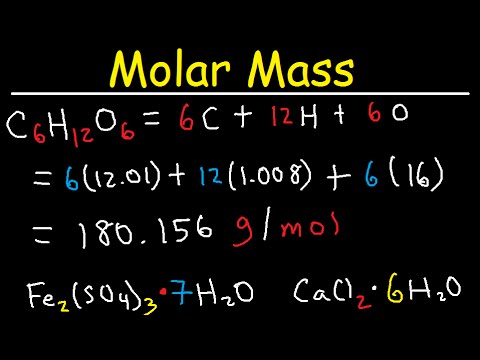 Source: youtube.com
Source: youtube.com
1 gram equivalent of HCl 1 mole of HCl. 30 mol3646 gmol 109 g HCl. Of moles x Avogadro number948 x 6022 x 1023. 90 M HCl 90 moles of HCl per liter of solution. 0012 molesmLx mL 01 moles x 010012 833 But r.
 Source: onlinemathlearning.com
Source: onlinemathlearning.com
Moles of solute liters of solution Molarity Moles of solute 050 L 60 M 30 mol of HCl. So the mass of water present 100 - 20 grams 80 grams. Therefore the number of moles of H 00125 moles. Zn s 2 HCl aq ZnCl2 aq H2 g For the calculations in this module the molar mass of an element will be rounded to the hundredths place 001 g. Molarity moles of solute volume of solution in liters If you know two of these quantities you can find.
 Source: pinterest.com
Source: pinterest.com
Mass HCl no. Molarity 301 M. Consider a 36 HCl solution containing 1000 g ie. A 361 HCl by mass. Now we have to convert the 30 mol of HCl into grams of HCl.
 Source: pinterest.com
Source: pinterest.com
-Remember that the sum of mole fractions of all the components of mixture or a solution is always equal to 1. Percent of water in the solution 100 - 36 64. If HCl solution has a high concentration such 01 001 mol dm-3 pH value is increased by 1 when concentration is reduced from 10 times. 1 mole is equal to 1 moles HCl or 3646094 grams. Of moles x Avogadro number948 x 6022 x 1023.
 Source: pinterest.com
Source: pinterest.com
1 kg of water. MgCl2 H2From the equation 1mole of Mg required 2moles of HCl Therefore 15moles of Mg will require 15 x 2 3 mo. Molecular weight of HCl or grams This compound is also known as Hydrochloric Acid. This compound is also known as Hydrochloric Acid. Now we have to convert the 30 mol of HCl into grams of HCl.
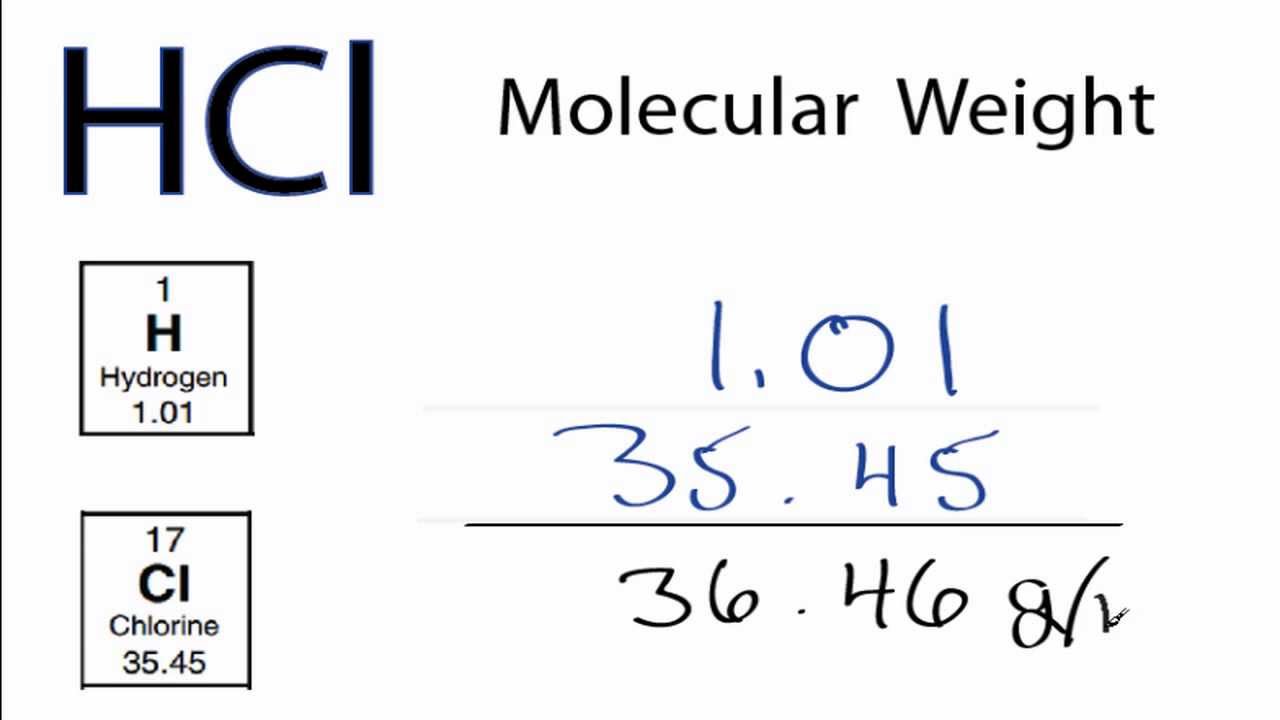 Source: youtube.com
Source: youtube.com
Concentrated HCl is 12 M which means 12 molesL or 0012 molesmL. Mass of HCl in the solution 1000 g 3664 5625 g. Molarity 301 M. Of moles x Avogadro number948 x 6022 x 1023. The molarity of concentrated hydrochloric acid HCl is 118 M and its density is 1190 gmL.
 Source: youtube.com
Source: youtube.com
1 mole is equal to 1 moles HCl or 3646094 grams. What is the mass of one mole of elemental oxygen O2. 0012 molesmLx mL 01 moles x 010012 833 But r. What mass of 60 HCl by mass is required to completely react with 02 mol of zinc and what volume of H 2 will be produced at STP. Moles of solute liters of solution Molarity Moles of solute 050 L 60 M 30 mol of HCl.
 Source: in.pinterest.com
Source: in.pinterest.com
100794 35453 Percent composition by element. This can be done by multiplying 30 mol by the molecular weight of HCl which is 3646 gmol. The problem requires that you determine the mass of hydrochloric acid HCl needed for the reaction to occur. B mole fraction 0219. To prepare 1 L of 05 N HCl solution you have to take 05 gram equivalent of pure HCl.
 Source: testbook.com
Source: testbook.com
30 mol3646 gmol 109 g HCl. Mass of HCL given346kgs 3460 grams of HCL No. Molarity moles of solute volume of solution in liters If you know two of these quantities you can find. Molar mass of HCl 3646094 gmol. Find the unknown concentration of KOH.
 Source: slidetodoc.com
Source: slidetodoc.com
The SI base unit for amount of substance is the mole. You can view more details on each measurement unit. Molarity 301 M. 90 M HCl 90 moles of HCl per liter of solution. 36461 gmol CAS Number.
 Source: khanacademy.org
Source: khanacademy.org
Zn s 2 HCl aq ZnCl2 aq H2 g For the calculations in this module the molar mass of an element will be rounded to the hundredths place 001 g. If HCl solution has a high concentration such 01 001 mol dm-3 pH value is increased by 1 when concentration is reduced from 10 times. Molar Mass Molecular Weight and Elemental Composition Calculator Enter a chemical formula to calculate its molar mass and elemental composition. B the mole fraction of HCl and c the molality of concentrated HCl. Molar mass of HCl 10 355 gmol 365 gmol.
 Source: pinterest.com
Source: pinterest.com
The SI base unit for amount of substance is the mole. Therefore the number of moles of HCl number of moles of H. To prepare 1 L of 05 N HCl solution you have to take 05 gram equivalent of pure HCl. What mass of 60 HCl by mass is required to completely react with 02 mol of zinc and what volume of H 2 will be produced at STP. Molar mass of HCl is 364609 gmol.
 Source: nagwa.com
Source: nagwa.com
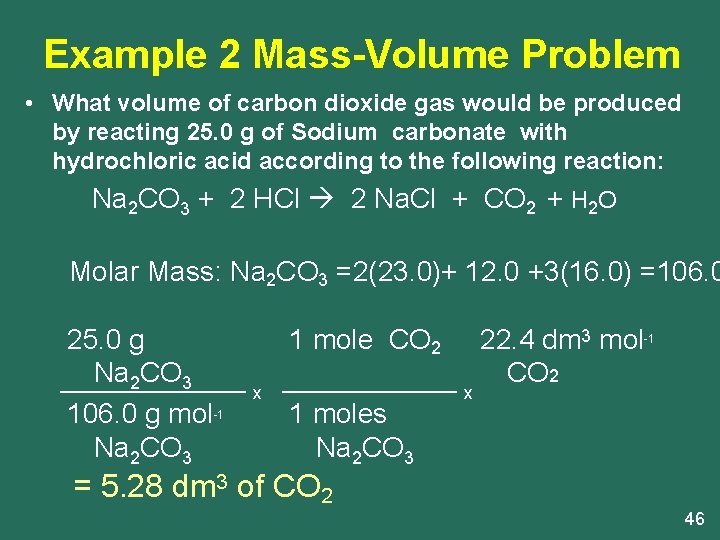
Mol HCl x molar mass HCl 0282 x 1008 3545 103 g. Molar Mass Molecular Weight and Elemental Composition Calculator Enter a chemical formula to calculate its molar mass and elemental composition. Moles of HCl vol x conc 25 x 01 00025 1000 1000 moles of NaOH moles of HCl 00025 volume of NaOH moles x1000 00025 x 1000 5cm 3 conc 05 15. To prepare 1 L of 05 N HCl solution you have to take 05 gram equivalent of pure HCl. You can view more details on each measurement unit.
 Source: wikihow.com
Source: wikihow.com
Every mole of HCl will produce one mole of H. What is the mass of one mole of elemental oxygen O2. HCl molecular weight. Designated by a capital M molL 60 M HCl 60 moles of HCl per liter of solution. A 01 M solution of HCl has 01 molesL.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title what is the mass of 01 mole of hcl by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.