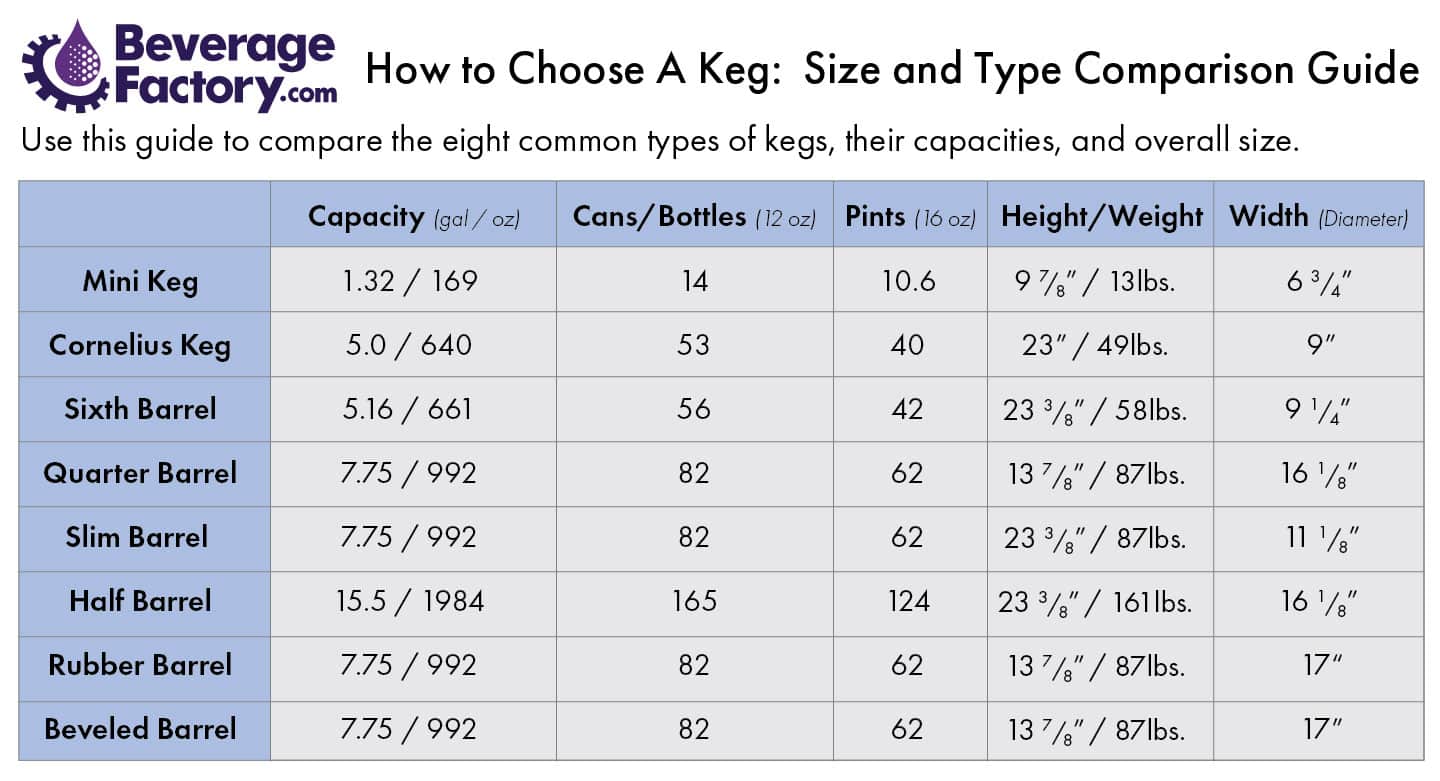
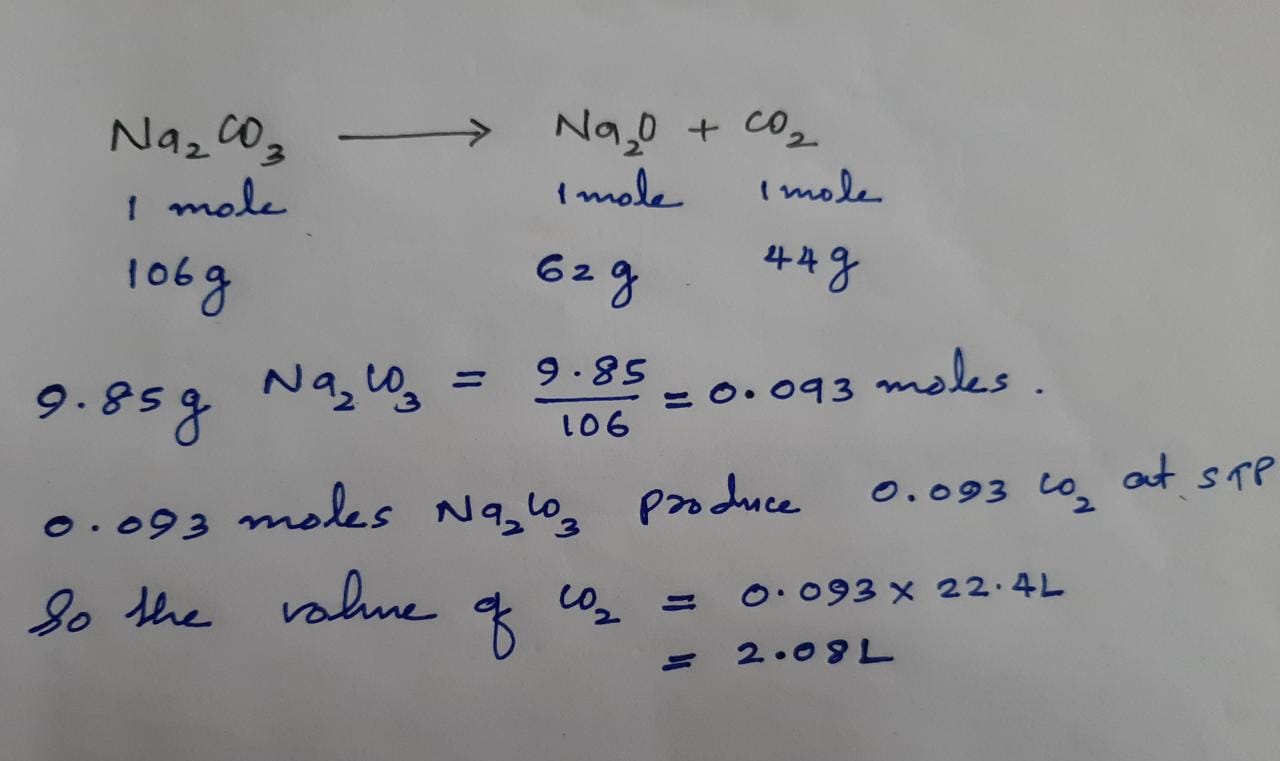Your What is the mass in grams of one mole of h2o images are available. What is the mass in grams of one mole of h2o are a topic that is being searched for and liked by netizens now. You can Find and Download the What is the mass in grams of one mole of h2o files here. Get all royalty-free photos and vectors.
If you’re looking for what is the mass in grams of one mole of h2o images information linked to the what is the mass in grams of one mole of h2o interest, you have visit the right site. Our website frequently gives you hints for refferencing the maximum quality video and picture content, please kindly search and find more informative video content and graphics that fit your interests.
What Is The Mass In Grams Of One Mole Of H2o. The molar mass of hydrogegn MH is 1008gmol and of oxygen MO is 15999 gmol so the molar mass ofwater is calculated as follows. 1 mole is equal to 1 moles H20 or 201588 grams. They do not affect the number of significant figures. In a sense the formula means H H O.
 The Mole Stoichiometry Ppt Download From slideplayer.com
The Mole Stoichiometry Ppt Download From slideplayer.com
3 rows H2O molecular weight. Now water has a molar mass of 18015 g mol1. The molecular formula for Water is H2O. If 18 grams are current in 1 mole of H2O how a lot mass is current in 126 moles of water. If 18 grams are present in 1 mole of H2O how much mass is present in 126. Molar mass of H2O is 1801528 000044 gmolOne mole of H 2 O is made up of 2 moles of hydrogen atoms and 1 mole of oxygen atom.
1 grams H2O 0055508435061792 mole using the molecular weight calculator and the molar mass of H2O.
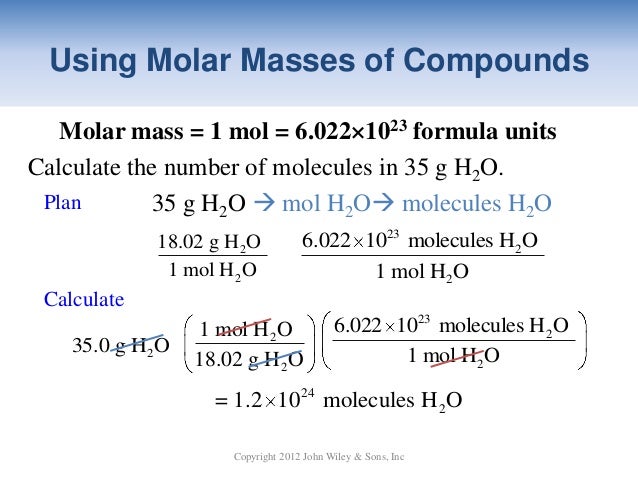
So every time your sample of water has a mass of 18015 g you can say for a fact that it contains 1 mole of water. How many moles of H2O are contained in 2199 g of water. They do not affect the number of significant figures. 1 mole is equal to 1 moles H2O or 1801528 grams. So that means a mole of water is 18 grams 1 1 16 and a mole of carbon dioxide is 44 grams. The molar mass of water is 1802 gmol.
 Source: slideshare.net
Source: slideshare.net
The volume occupied by one mole of a gas at standard correlation. The atomic mass of O 16. The number of moles of propane gas C3H8 are contained in 11 grams of the gas at standard conditions. This compound is also known. The SI base unit for amount of substance is the mole.
 Source: slideplayer.com
Source: slideplayer.com
MH2O 2 x MH 1 x M. The atomic mass of H2O is 210 160 180Amount of H2O mass of pure. 128 moles 12818 2304 grams. This compound is also known. The number of atoms is an exact number the number of mole is an exact number.
 Source: pinterest.com
Source: pinterest.com
Do a quick conversion. How many moles in 75g water. This implies 1 mole of hydrogen weighs 10079 grams and 1 mole of oxygen weighs 159994 grams. MH2O 2 x MH 1 x M. Mass of 1 mole of water.
 Source: slideplayer.com
Source: slideplayer.com
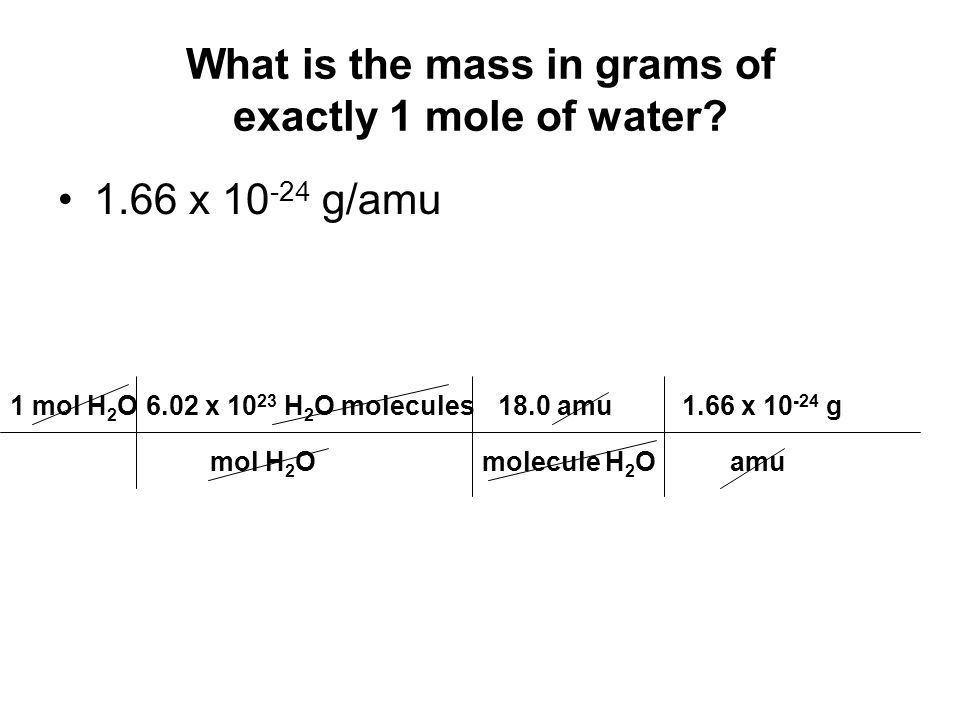
You can view more details on each measurement unit. 3 rows H2O molecular weight. The weight of 1 molecule of water is 18015gmolAs each water molecule consists of 2 atoms of hydrogen and 1 atom of oxygen. The molecular formula of water is H2O. The common mass of 1 mole of H 2 O is 1802 grams.
 Source: bartleby.com
Source: bartleby.com
Molar Mass Molecular Weight and Elemental Composition Calculator Enter a chemical formula to calculate its molar mass and elemental composition. 1 mole is equal to 1 moles Water or 1801528 grams. One mole of atoms is the atomic mass of the element in grams. They do not affect the number of significant figures. In this case being.
 Source: clutchprep.com
Source: clutchprep.com
The SI base unit for amount of substance is the mole. The molecular formula of water is H 2O. The molecular formula for Water is H2O. The average mass of one mole of H2O is 1802 grams. You can view more details on each measurement unit.
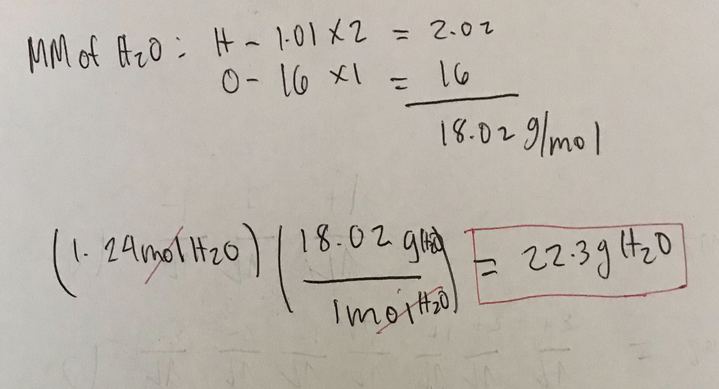 Source: clutchprep.com
Source: clutchprep.com
Notice that the molar mass and the formula mass are numerically the same. G mol1 MgSO4 H2O 138. If 10 g samples of each compound were dehydrated which sample would lose the greatest mass of water. The molecular formula of water is H2O. The atom that serves as the relative standard of determining mass and the number of particles in a mole.
 Source: slideplayer.com
Source: slideplayer.com
The number of atoms is an exact number the number of mole is an exact number. 1 mole H2O 180 gmol H2O 180 g H2O Molar mass H2O 210 1160 gmol How many moles of water molecules h2o are present in a 27 rams sample of water. Molar Mass Molecular Weight and Elemental Composition Calculator Enter a chemical formula to calculate its molar mass and elemental composition. The molar mass of hydrogegn MH is 1008gmol and of oxygen MO is 15999 gmol so the molar mass ofwater is calculated as follows. The mass of two moles of propane gas C3H8.
 Source: slidetodoc.com
Source: slidetodoc.com
In a sense the formula means H H O. The average mass of one H2O molecule is 1802 amu. The number of atoms is an exact number the number of mole is an exact number. Then you can apply the following rule of three. The weight of 1 molecule of water is 18015gmolAs each water molecule consists of 2 atoms of hydrogen and 1 atom of oxygen.
 Source: slideplayer.com
Source: slideplayer.com
1 mole elemental oxygen16 grams O1 mole 16 grams. The atom that serves as the relative standard of determining mass and the number of particles in a mole. Then convert from moles to grams. 128 moles 12818 2304 grams. 1 mole H2O 180 gmol H2O 180 g H2O Molar mass H2O 210 1160 gmol How many moles of water molecules h2o are present in a 27 rams sample of water.
 Source: slideplayer.com
Source: slideplayer.com
Note that rounding errors may occur so always check the results. H₂O 21 gmole 16 gmole 18 gmole. The number of atoms is an exact number the number of mole is an exact number. This implies 1 mole of hydrogen weighs 10079 grams and 1 mole of oxygen weighs 159994 grams. This tells you that 1 mole of water has a mass of 18015 g.
 Source: slideplayer.com
Source: slideplayer.com
The number of atoms is an exact number the number of mole is an exact number. Notice that the molar mass and the formula mass are numerically the same. How many moles in 75g water. GivenMass of magnesium hydroxide 450gVolume of the solution 65LThe moles of the magnesium hy. This tells you that 1 mole of water has a mass of 18015 g.
 Source: slideplayer.com
Source: slideplayer.com
The molecular formula of water is H2O. In this case being. The molecular formula for Water is H2O. 1 mole of H2O 2116 18 g. The average mass of one H2O molecule is 1802 amu.
 Source: slideplayer.com
Source: slideplayer.com
Notice that the molar mass and the formula mass are numerically the same. They do not affect the number of significant figures. In this case being. Use this page to learn how to convert between moles H2O and gram. The weight of 1 molecule of water is 18015gmolAs each water molecule consists of 2 atoms of hydrogen and 1 atom of oxygen.
 Source: youtube.com
Source: youtube.com
Molar mass of H2O is 1801528 000044 gmolOne mole of H 2 O is made up of 2 moles of hydrogen atoms and 1 mole of oxygen atom. Its big number though. G mol1 MgSO4 H2O 138. So that means a mole of water is 18 grams 1 1 16 and a mole of carbon dioxide is 44 grams. If 18 grams are present in 1 mole of H2O how much mass is present in 126.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title what is the mass in grams of one mole of h2o by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.