Your What is the mass in grams of 1 molecule of caffeine images are available in this site. What is the mass in grams of 1 molecule of caffeine are a topic that is being searched for and liked by netizens now. You can Download the What is the mass in grams of 1 molecule of caffeine files here. Download all royalty-free photos.
If you’re looking for what is the mass in grams of 1 molecule of caffeine pictures information connected with to the what is the mass in grams of 1 molecule of caffeine topic, you have visit the ideal blog. Our website always gives you suggestions for downloading the maximum quality video and image content, please kindly surf and locate more enlightening video content and images that match your interests.
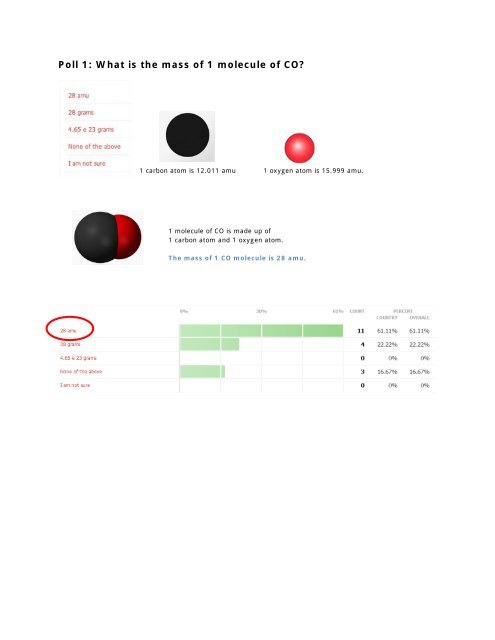
What Is The Mass In Grams Of 1 Molecule Of Caffeine. Therefore the mass of 00150 mol of caffeine 00150194 291 g. What is the mass in gram of one molecule of caffeine c8h10n4o2. Computed by PubChem 21 PubChem release 20210507 Monoisotopic Mass. 1 u is equal to 112 the mass of one atom of carbon-12.
 What Is The Mass In Grams Of One Molecule Of Caffeine Ch H02 Brainly In From brainly.in
What Is The Mass In Grams Of One Molecule Of Caffeine Ch H02 Brainly In From brainly.in
Use an online molecular weight calculator to work out the molar mass of chemical compounds by following steps. What is the mass in grams of one molecule of caffeine. 1201078 10079410 1400674 1599942 Percent composition by element. 22 x10-4 24 IM. We assume we have a 100g sample of caffeine and so 4948 Carbon is equal to 4948 g of Carbon in the sample 515 Hydrogen is equal to 515 g of Hydrogen in the sample and so on. The mass of 1 mol of caffeine - 194 g.
Mol massmolecular mass.
Mol massmolecular mass. Determine the number of moles of each type of atom present. Element mass in g m. What is the percentage of carbon hydrogen and oxygen. Molecular mass molecular weight is the mass of one molecule of a substance and is expressed in the unified atomic mass units u. 4948g 1201 g mol 412mol of Carbon.
 Source: pinterest.com
Source: pinterest.com
What is the percentage of carbon hydrogen and oxygen. The molecular formula for Caffeine is C8H10N4O2. Caffeine has a molar mass of 195 grams per mole. Computed by PubChem 21 PubChem release 20210507 Topological Polar Surface Area. Molecular weight of Caffeine or mol.
 Source: pinterest.com
Source: pinterest.com
What is the molar mass of caffeine C 8 H 10 N 4 O 2. What is the percentage of carbon hydrogen and oxygen in methanol. Molecular weight of Na2CO3 or grams This compound is also known as Sodium CarbonateThe SI base unit for amount of substance is the mole. Computed by PubChem 21 PubChem release 20210507 Topological Polar Surface Area. 1 u is equal to 112 the mass of one atom of carbon-12.
 Source: toppr.com
Source: toppr.com
C8H10N4O2 molecular weight. In 1 mol of caffeine mass of C is -. What is the molar mass of caffeine C 8 H 10 N 4 O 2. So one mole of water 1600g 2016 g 1802 g. 522 of Carbon 130 of Hydrogen and 348 of Oxygen.
 Source: toppr.com
Source: toppr.com
22 x10-4 24 IM. Mass 44 g mol 60221023 molecules mol 7310-23 g. So one mole of water 1600g 2016 g 1802 g. Caffeine has a molar mass of 195 grams per mole. Convert grams C8H10N4O2 to moles or moles C8H10N4O2 to grams.
 Source: toppr.com
Source: toppr.com
The molar mass of caffeine is. Note that rounding errors may occur so always check the results. Use an online molecular weight calculator to work out the molar mass of chemical compounds by following steps. 165 percent Find the empirical and molecular formulas of caffeine. Definitions of molecular mass molecular weight molar mass and molar weight.
 Source: pinterest.com
Source: pinterest.com
Mol massmolecular mass. Molar mass CO2 440 gmol. What is the mass in gram of one molecule of caffeine c8h10n4o2. 1 mole is equal to 1 moles Caffeine or 1941906 grams. Molar mass is the mass in grams of 1 mol of substance The number of particles in a mole is equal to 6022 10 23.

And the total is 194193 g which is the molar mass of caffeine. 522 of Carbon 130 of Hydrogen and 348 of Oxygen. So mass molmolecular mass. The molecular formula for Caffeine is C8H10N4O2. What is the percentage of carbon hydrogen and oxygen in methanol.
 Source: pinterest.com
Source: pinterest.com
Obtain the mass of each element present in grams. Computed by PubChem 21 PubChem release 20210507 Topological Polar Surface Area. The moles of caffeine in a given amount of coffee is calculated by multiplying the number of grams of coffee by the molecular weight of caffeine. Computed by PubChem 21 PubChem release 20210507 Monoisotopic Mass. If you have 125 grams of molecules with a molecular weight of 1341 gmol how many moles do you have.
 Source: pinterest.com
Source: pinterest.com
More information from the unit converter You can view more details on each measurement unit. 22 x10-4 24 IM. Divide the number of moles of each element by the smallest number of moles. Caffeine has a molecular weight of 194. How Molar Mass Calculator Works.
 Source: in.pinterest.com
Source: in.pinterest.com
So mass molmolecular mass. Also Know what is the molar mass of caffeine c4h5n2o. Obtain the mass of each element present in grams. Molecular weight of Na2CO3 or grams This compound is also known as Sodium CarbonateThe SI base unit for amount of substance is the mole. The molar mass of caffeine is 19419g mol.
 Source: doubtnut.com
Source: doubtnut.com
The SI base unit for amount of substance is the mole. Therefore the mass of 00150 mol of caffeine 00150194 291 g. The molar mass of caffeine is. 4948g 1201 g mol 412mol of Carbon. What is the mass in grams of one molecule of caffeine.
 Source: pinterest.com
Source: pinterest.com
Molar mass is the mass in grams of 1 mol of substance The number of particles in a mole is equal to 6022 10 23. So mass molmolecular mass. 1 grams Caffeine is equal to 00051495798457804 mole. C8H10N4O2 molecular weight. So one mole of water 1600g 2016 g 1802 g.
 Source: yumpu.com
Source: yumpu.com
More information from the unit converter You can view more details on each measurement unit. Molecular mass molecular weight is the mass of one molecule of a substance and is expressed in the unified atomic mass units u. Examples of molecular weight computations. Now we take those numbers and convert them to moles of the element we are dealing with so. Caffeine a stimulant found in coffee has the mass composition of C 4948 H 519 N 2885 O 1648.
 Source: toppr.com
Source: toppr.com
22 x10-4 24 IM. What is the molar mass of caffeine C 8 H 10 N 4 O 2. So one mole of water 1600g 2016 g 1802 g. Determine the number of moles of each type of atom present. 1201078 10079410 1400674 1599942 Percent composition by element.
 Source: brainly.in
Source: brainly.in
Divide the number of moles of each element by the smallest number of moles. Molar mass is the mass in grams of 1 mol of substance The number of particles in a mole is equal to 6022 10 23. The molecular formula of Caffeine is C₈H₁₀N₄O₂. More information from the unit converter You can view more details on each measurement unit. Element mass in g m.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title what is the mass in grams of 1 molecule of caffeine by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






