Your What is the mass in grams of 1 mole of o2 gas images are available. What is the mass in grams of 1 mole of o2 gas are a topic that is being searched for and liked by netizens today. You can Get the What is the mass in grams of 1 mole of o2 gas files here. Get all free vectors.
If you’re searching for what is the mass in grams of 1 mole of o2 gas images information related to the what is the mass in grams of 1 mole of o2 gas keyword, you have pay a visit to the ideal site. Our site frequently gives you suggestions for seeking the maximum quality video and picture content, please kindly search and find more informative video articles and graphics that match your interests.
What Is The Mass In Grams Of 1 Mole Of O2 Gas. O 2 is a diatomic molecule. You can view more details on each measurement unit. 5 moles Oxygen to grams 79997 grams. 1 moles Oxygen to grams 159994 grams.
 Moles What Is Molar Mass Molar Mass Is From slidetodoc.com
Moles What Is Molar Mass Molar Mass Is From slidetodoc.com
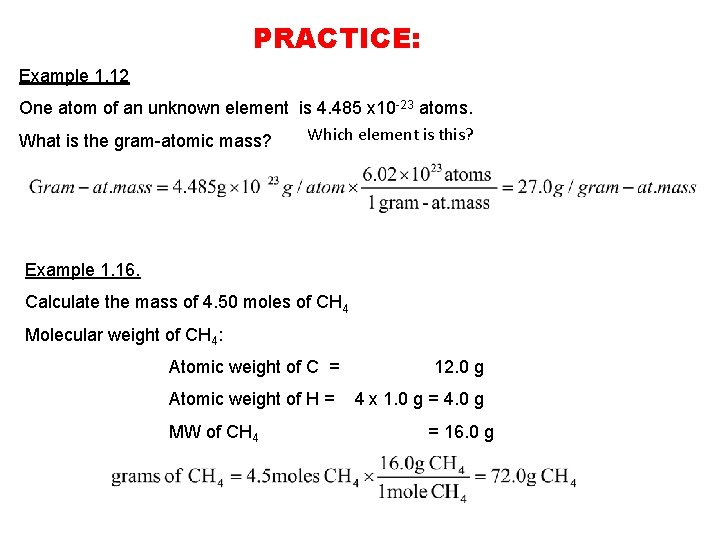
Molecular weight of O2 or grams. 1 mole of any gas at stp is 224 liters. 4 moles Oxygen to grams 639976 grams. O is 16gmole so O2 is 32gmole. Its been 15 years since I graduated HS and I. Divide the amount you have by the molar weight to obtain the number of moles in the sample.
4 moles Oxygen to grams 639976 grams.
O is 16gmole so O2 is 32gmole. 7 moles Oxygen to grams 1119958 grams. You can view more details on each measurement unit. The percent composition by mass of nitrogen in NH4OH gram-formula mass. 32 gmol and the molar mass of nitrogen gas is. So the answer is 639976 g.
 Source: studylib.net
Source: studylib.net
One mole of oxygen is 159994 g but because oxygen is a diatomic molecule it is bonded with itself. 3 moles Oxygen to grams 479982 grams. Use this page to learn how to convert between moles Co2 and gram. 1 grams O2 0031251171918947 mole using the molecular weight calculator and the molar mass of O2. 4 moles Oxygen to grams 639976 grams.
 Source: slideplayer.com
Source: slideplayer.com
5032 15625 moles. From here the answer is simple. 16 grams or 32 grams depending on what youre looking atThe mass of one mole of oxygen atoms is about 16 grams but oxygen gas exists as. So 159994 g 2 319988 g. O is 16gmole so O2 is 32gmole.
 Source: pinterest.com
Source: pinterest.com
Oxygen has a molar mass of 159994 gmol and nitrogen has a molar mass of 140067 gmol. 1008 gramsmole Hydrogen 1 mole6022x1023 atoms 167 x 10-24 grams. Mass of one molecule of oxygen. Its been 15 years since I graduated HS and I. 1 grams O2 0031251171918947 mole using the molecular weight calculator and the molar mass of O2.
 Source: slidetodoc.com
Source: slidetodoc.com
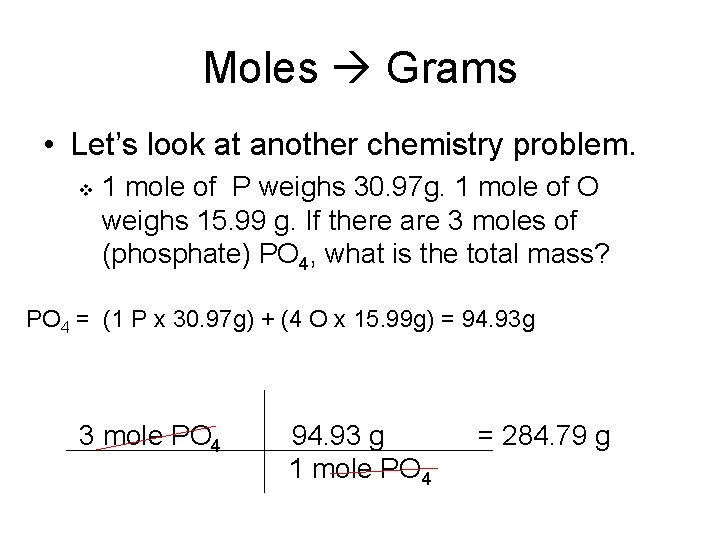
Molar mass of O2 319988 gmol. Convert grams O2 to moles or moles O2 to grams. You can view more details on each measurement unit. Oxygen gas is diatomic meaning two atoms of 16 gmol each so 1 mole of O2 has a mass of 32 grams. So three moles of oxygen atoms would have a mass of 48g.
 Source: toppr.com
Source: toppr.com
1 mole is equal to 1 moles O2 or 319988 grams. Molecular weight of O2 or grams. These numbers are either in atomic mass units amu or in grams per mole of atoms. 1 mole is equal to 1 moles O2 or 319988 grams. The volume occupied by one mole of a gas at standard correlation.
 Source: slideplayer.com
Source: slideplayer.com
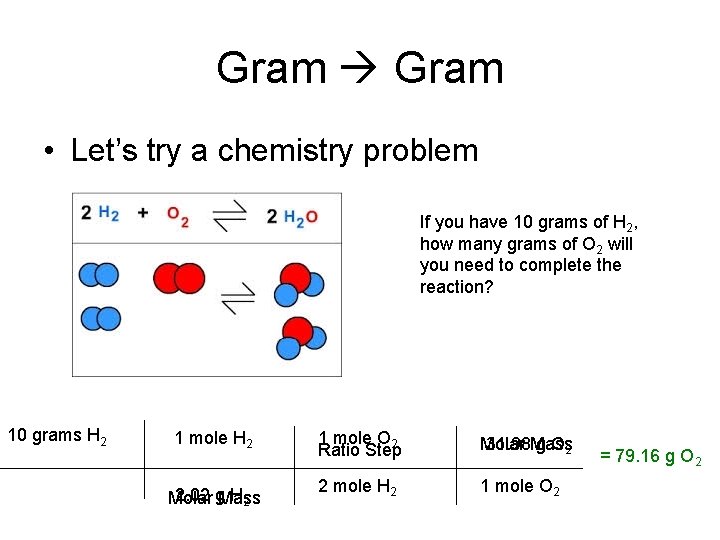
One mole of oxygen gas which has the formula O 2 has a mass of 32 g and contains 602 X 10 23 molecules of oxygen but 1204 X 10 23 2 X 602 X 10 23 atoms because each molecule of oxygen contains two oxygen atoms. One mole of atoms of oxygen has a mass of 16 g as 16 is the atomic weight of oxygen and contains 602 X 10 23 atoms of oxygen. We assume you are converting between moles O2 and gram. 1 mole of any gas at STP 224 Liters 5 g CO_2g 5 g44gmole 0114 mole CO_2g Volume of 0114 mole CO_2g 0114 mole224 Lmole 255 Liters CO_2 g at STP. 1 grams O2 0031251171918947 mole using the molecular weight calculator and the molar mass of O2.
 Source: slideplayer.com
Source: slideplayer.com
O is 16gmole so O2 is 32gmole. What is the total number of moles of atoms contained in 1 mole of NH3. The answer is 0031251171918947. 8 moles Oxygen to grams 1279952 grams. Oxygen gasA few things to consider when finding the molar mass for O2- make sure you have the correct chem.

The SI base unit for amount of substance is the mole. 15625 224 35 liters. Divide the amount you have by the molar weight to obtain the number of moles in the sample. Do a quick conversion. Which sample contains a total of 60 x 1023 atoms.
 Source: slidetodoc.com
Source: slidetodoc.com
The SI base unit for amount of substance is the mole. One mole of O2 has approximately the same mass as one mole of. 6 moles Oxygen to grams 959964 grams. Explanation of how to find the molar mass of O2. 1599942 Percent composition by element.
 Source: youtube.com
Source: youtube.com
When the equation H2O2 — H2O O2 is completely balanced the sum of all the coefficients will be. One mole of oxygen atoms has a mass of 16g. 1 mole is equal to 1 moles Co2 or 1178664 grams. That said to find the mass of one ATOM we need to convert from moles to atoms as follows. 3 moles Oxygen to grams 479982 grams.
 Source: pinterest.com
Source: pinterest.com
1 grams O2 0031251171918947 mole using the molecular weight calculator and the molar mass of O2. 1599942 Percent composition by element. O_2 has a mass of 178g. Now mass can be calculated by. Of moles Given Volume 224 Litre provided that gas is at STP 112 Litre 224 Litre.
 Source: slideplayer.com
Source: slideplayer.com
So the answer is 639976 g. 1 moles Oxygen to grams 159994 grams. 1 mole of any gas at STP 224 Liters 5 g CO_2g 5 g44gmole 0114 mole CO_2g Volume of 0114 mole CO_2g 0114 mole224 Lmole 255 Liters CO_2 g at STP. Was this answer helpful. 1 mole is equal to 1 moles O2 or 319988 grams.
 Source: slideplayer.com
Source: slideplayer.com
Mass of O 2 molecule 2 16 32 amu. You can view more details on each measurement unit. How is the molar mass of oxygen gas calculated. The atom that serves as the relative standard of determining mass and the number of particles in a mole. The number of moles of propane gas C3H8 are contained in 11 grams of the gas at standard conditions.
 Source: toppr.com
Source: toppr.com
Oxygen has a molar mass of 159994 gmol and nitrogen has a molar mass of 140067 gmol. 1 mole is equal to 1 moles Co2 or 1178664 grams. When the equation H2O2 — H2O O2 is completely balanced the sum of all the coefficients will be. The gram formula mass of NH42CO3 is. We assume you are converting between moles O2 and gram.
 Source: pinterest.com
Source: pinterest.com
1 mole is equal to 1 moles Co2 or 1178664 grams. The number of moles of propane gas C3H8 are contained in 11 grams of the gas at standard conditions. The gram formula mass of NH4Cl is. How much mass does 1 mole of O2 gas have. Oxygen gas is diatomic meaning two atoms of 16 gmol each so 1 mole of O2 has a mass of 32 grams.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title what is the mass in grams of 1 mole of o2 gas by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.