Your What is the density of one mole of water images are available. What is the density of one mole of water are a topic that is being searched for and liked by netizens today. You can Download the What is the density of one mole of water files here. Get all royalty-free vectors.
If you’re looking for what is the density of one mole of water pictures information connected with to the what is the density of one mole of water topic, you have come to the ideal site. Our website frequently provides you with hints for seeing the highest quality video and picture content, please kindly search and find more informative video content and images that fit your interests.
What Is The Density Of One Mole Of Water. Moles H3PO4 870 g 979937 gmol 08878 mol. Molality 08878 mol 00130 kg 683 molal. Density of water is equal to 1 000 kgm³. The mass m of 1 mol of He is 4003 g.
 The Mole 1 Dozen 1 Century 1 Pair 1 Mole X There Are Exactly 12 Grams Of Carbon 12 In One Mole Of Carbon Ppt Download From slideplayer.com
The Mole 1 Dozen 1 Century 1 Pair 1 Mole X There Are Exactly 12 Grams Of Carbon 12 In One Mole Of Carbon Ppt Download From slideplayer.com
Weight of water 20158 g 159994 g. 16 O1 H 1 H 18 We then take 1000g 18gmole 555555 moles. Therefore the mass of one mole of water 18 Grams. The density of the answer is 104 gmL. This is useful if you want to envision the distance between molecules in different samples. The density of water is 1 gml.
Density of water lbft 3.
The mass of water in the solution is. 867 m 3 significant digits. V m 18 cm 3 mol. Since the density 118gmL then 1 L 1000 mL will have a. Volume of 1 molecule of water 18 6022 10 23 Volume of. Volume of 1 molecule of water V m N A.
 Source: pinterest.com
Source: pinterest.com
Moles H3PO4 870 g 979937 gmol 08878 mol. As all gases that are behaving ideally have the same number density they will all have the same molar volume. What is the density in gl of helium gas at STP. For instance if you have a sample of liquid water it has a mass density of 1 g mL1. At 25C 77F or 29815K at standard atmospheric pressure.
 Source: slideplayer.com
Source: slideplayer.com
So our liter has 1000 grams of mass. For instance if you have a sample of liquid water it has a mass density of 1 g mL 1. In a sense the formula means H H O. The volume of 1 mole pure ethanol is 580 ml and the volume of 1 mole pure water is 180 ml. The atomic mass of H 1.
 Source: pinterest.com
Source: pinterest.com
Therefore 1 litre of water 1000 ml would weigh 1000 g. We know Water density is 1 grammL. 867 m 3 significant digits. The mass of one mole of any atommolecule is equal to its atomic molecular mass in grams. The molarity of something is the number of moles of that thing per 1000 ml volume.
 Source: slideplayer.com
Source: slideplayer.com
V displaystyle tilde V of a substance is the volume occupied by one mole of it at a given temperature and pressure. Weight of water 2 10079 g 159994 g. And since the molar mass of water is 18 g per mol 18 gmol we can convert the 1000 g of water to moles of water by multiplying 1000 g of water by the ratio 1 mol18 g. It is equal to the molar mass M divided by the mass density ρ. Liquids often have densities of about 10 gcm 3 and indeed fresh water has a density of 10 gcm 3.
 Source: slideplayer.com
Source: slideplayer.com
If the gas to be absorbed contains 55 SO 3. 100 mole NaCl. 867 m 3 significant digits. Molar mass of water M 18 gmol. Grams have a relationship with moles via the molar mass of a molecule.
 Source: pinterest.com
Source: pinterest.com
Quantity mass density Quantity of 1 mole of water 18 g 1 gml Quantity of 1. The mass of one mole of any atommolecule is equal to its atomic molecular mass in grams. Moles H3PO4 870 g 979937 gmol 08878 mol. Density of water ρ 1gcm 3. It is equal to the molar mass M divided by the mass density ρ.
 Source: youtube.com
Source: youtube.com
Density is mass divided by volume ρmv and water was used as the basis for establishing the metric unit of mass which means a cubic centimeter 1cm 3 of water weighs one gram 1g. However 1 mole water mixed with 1 mole ethanol does not result in 580 ml 180 ml or 760 ml but rather 743. It is equal to the molar mass M divided by the mass density ρ. 100 1364 98636 g H 2 O. At STP this will be 224 L.
 Source: pinterest.com
Source: pinterest.com
Volume of 1 molecule of water 18 6022 10 23 Volume of. Therefore it follows that 1000 mL one liter of water will contain 1000 g of water molecules. V displaystyle tilde V of a substance is the volume occupied by one mole of it at a given temperature and pressure. For instance if you have a sample of liquid water it has a mass density of 1 g mL 1. Density of water kgm 3.
 Source: pinterest.com
Source: pinterest.com
What is volume occupied by 1 molecule of water. Volume of 1 molecule of water V m N A. Therefore 1 litre of water 1000 ml would weigh 1000 g. For instance if you have a sample of liquid water it has a mass density of 1 g mL1. What is the density in gl of helium gas at STP.
 Source: youtube.com
Source: youtube.com
V m M ρ displaystyle V_ text m frac M rho. And since the molar mass of water is 18 g per mol 18 gmol we can convert the 1000 g of water to moles of water by multiplying 1000 g of water by the ratio 1 mol18 g. It is equal to the molar mass M divided by the mass density ρ. Hence it can be said that the volume V of 4003 g of He at STP is 22400 mL. 1 1 16 18.
 Source: slideplayer.com
Source: slideplayer.com
Solution prepared by dissolving 100 mole of NaCl in 100L of water. A solution that is 756 by mass NaNO 3 molar mass850 gmole in water molar mass180 gmole has a density of 109 gmL. What is the molality of a solution that has a mole fraction of 0102 KF molar mass581 gmole in water molar mass180 gmole. V m 18 cm 3 mol. The density of water is 1 gml.
 Source: chemistrygod.com
Source: chemistrygod.com
What is the molality of a solution that has a mole fraction of 0102 KF molar mass581 gmole in water molar mass180 gmole. The atomic mass of O 16. The molecular weight of water is. The volume of 1 mole pure ethanol is 580 ml and the volume of 1 mole pure water is 180 ml. Liquids often have densities of about 10 gcm 3 and indeed fresh water has a density of 10 gcm 3.
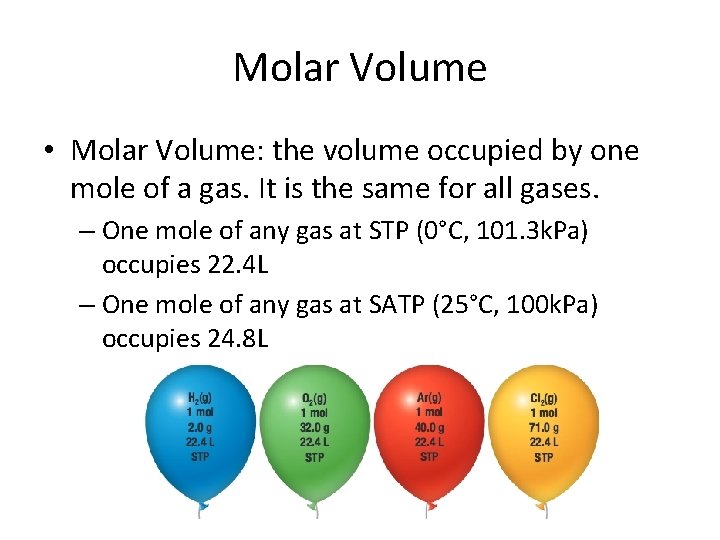 Source: slidetodoc.com
Source: slidetodoc.com
It is equal to the molar mass M divided by the mass density ρ. Weight of water 20158 g 159994 g. Grams have a relationship with moles via the molar mass of a molecule. Density of water kgm 3. On the other hand water and ethanol do not form an ideal solution.
 Source: slideplayer.com
Source: slideplayer.com
Moles H3PO4 870 g 979937 gmol 08878 mol. Volume of one molecule of water is. A solution that is 756 by mass NaNO 3 molar mass850 gmole in water molar mass180 gmole has a density of 109 gmL. What is the vapor pressure of a solution prepared by dissolving 1058 g naphthalene C10H8 Molar Mass 1282 gmol in 129 mL CS2 liquid Molar Mass 7614 gmol density 1261 gmL. Molar volume Vm Mρ.
 Source: slideplayer.com
Source: slideplayer.com
The atomic mass of O 16. The molecular weight of water is. Volume of 1 molecule of water V m N A. For instance if you have a sample of liquid water it has a mass density of 1 g mL1. Rocks often have a density around 3 gcm 3 and metals often have densities above 6 or 7 gcm 3.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title what is the density of one mole of water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






