Your What is meant by concentration in unit g mol 1 images are ready. What is meant by concentration in unit g mol 1 are a topic that is being searched for and liked by netizens today. You can Get the What is meant by concentration in unit g mol 1 files here. Get all free vectors.
If you’re searching for what is meant by concentration in unit g mol 1 images information related to the what is meant by concentration in unit g mol 1 interest, you have pay a visit to the right blog. Our website always gives you hints for refferencing the highest quality video and picture content, please kindly search and find more informative video articles and graphics that fit your interests.
What Is Meant By Concentration In Unit G Mol 1. 1 Units and concentrations 11 Commonly used concentration units Commonly concentration units are presented using units in the form of mass per volume eg. Where ε LCP and ε RCP are the molar extinction coefficients for LCP and RCP light respectively C molar concentration and l pathlength in centimeters. As you can see this number is so large that we can dissolve chemicals in. Finally calculate the concentration molarity Concentration dissolved moles total volume.
 Molarity 2 Molarity M This Is The Most Common Expression Of Concentration M Molarity Moles Of Solute Mol Liters Of Solution L Units Are Ppt Download From slideplayer.com
Molarity 2 Molarity M This Is The Most Common Expression Of Concentration M Molarity Moles Of Solute Mol Liters Of Solution L Units Are Ppt Download From slideplayer.com
As you can see this number is so large that we can dissolve chemicals in. Concentration 5556 mol 1 dm 3. If it were mol L-1 s-2 this would mean moles per litre per second per second. From mol dm-3 we know we have to calculate the molarity. Assuming the density of water is 100 gmL 1 liter of solution 1 kg and hence 1 mgL 1 ppm. Multiply 1 L X the density 154 gmL X 1000 mLL.
Concentration 5556 mol 1 dm 3.
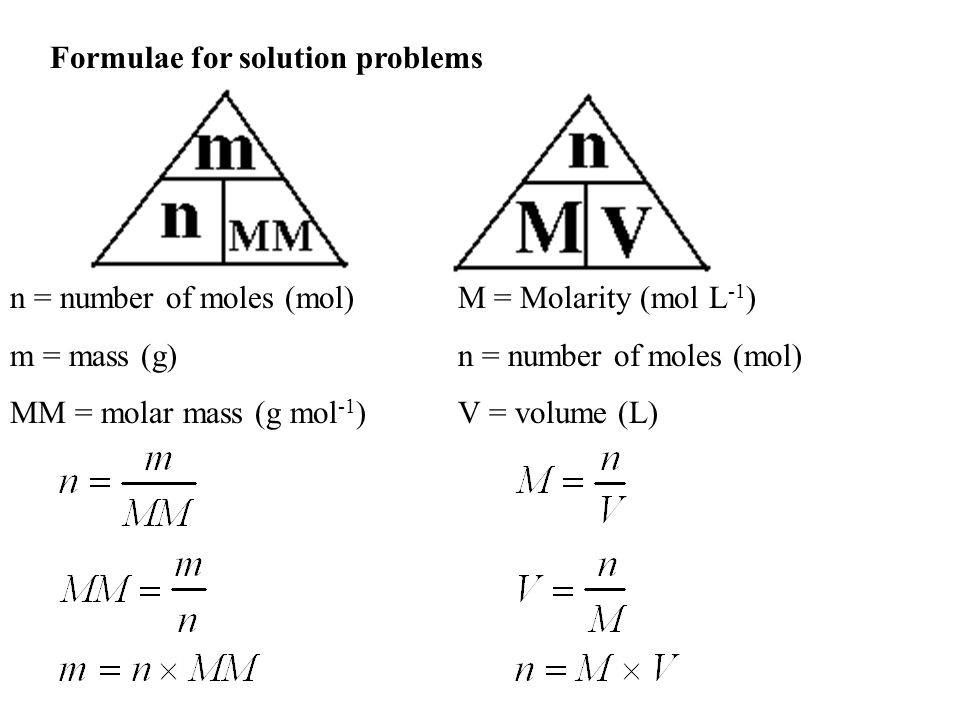
The mole the standard unit of amount of a substance mol the number of particles in a mole is known as Avogadros constant NA Avogadros constant has a value of 602 x 1023 mol-1. 1 g glucose dissolved in water to a final volume of 100 ml solution gives a 1 wv glucose solution. So your first example molL-1 s-1 is not correct - it would actually be written as mol L-1 s-1 OR molL s. The concentration of a solution can be calculated using. Concentration moles volume. To calculate concentration we use CnV where C is the molar concentration n is the number of moles and V is the volume of the solution.

M i is the mass of one mole of substance i. Milligrams per litre mgL mgL-1 for water samples or mass per mass eg. 1 ww salt in sand. Number of moles 1000 g 18 g mol-1. If it were mol L-1 s-2 this would mean moles per litre per second per second.
 Source: chemistrygod.com
Source: chemistrygod.com
Milligrams per litre mgL mgL-1 for water samples or mass per mass eg. The solute may be a solid ionic or covalent a gas or a liquid but is commonly a solid. Mole fraction χ moles of solutetotal moles Concentrations expressed as ppm and N are less familiar to most students at this stage. C eq z c. M i is the mass of one mole of substance i.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
One mole represents N A entities atoms molecules ions electrons where N A 60210 23 mol -1 is the Avogadro constant. Convert 25 moles to grams. Number of moles 1000 g 18 g mol-1. The concentration of a solution can be calculated using. It has units of g mol-1 or kg mol-1.
 Source: thebumblingbiochemist.com
Source: thebumblingbiochemist.com
1 ww salt in sand. As you can see this number is so large that we can dissolve chemicals in. Concentration of water 5556 mol dm-3. Concentration is the number of moles of a substance dissolved in one decimetre cubed dm3 which is 1000cm3 or 1 litre usually in pure water. Finally calculate the concentration molarity Concentration dissolved moles total volume.
 Source: ro.pinterest.com
Source: ro.pinterest.com
It is a well-known tabulated quantity unit. 1 So its quite easy to jump between molar and mass concentrations. Multiply 1 L X the density 154 gmL X 1000 mLL. M i is the mass of one mole of substance i. 25M means 25 moles of sulfuric acid per one liter of solution.
 Source: pinterest.com
Source: pinterest.com
For converting an equivalent concentration c eq into the corresponding molar concentration c divide the equivalent concentration by the valency z ie. The solute may be a solid ionic or covalent a gas or a liquid but is commonly a solid. Convert 25 moles to grams. This is a unit specific for. Conversion from molar extinction absorbance corrected for concentration to molar ellipticity uses a factor of 3298 θ 3298Δε.
 Source: pinterest.com
Source: pinterest.com
Specifically molarity is defined as. For converting an equivalent concentration c eq into the corresponding molar concentration c divide the equivalent concentration by the valency z ie. The molar mass of sulfuric acid is 9809 gmol. Number of moles 1000 g 18 g mol-1. This set of units is referred to as molarity and is a common measure of concentration for a solute dissolved in a solvent.
 Source: pinterest.com
Source: pinterest.com
What is meant by concentration in unit g mol 1. The molar mass of water is 18 g mol-1. The formula for density is d MV. An aqueous solution that contains 1 mol 342 g of sucrose in enough water to give a final volume of 100 L has a sucrose concentration of 100 molL or 100 M. Find the total mass of the solution.
 Source: thebumblingbiochemist.com
Source: thebumblingbiochemist.com
4B-1 Concentration of Solutions The molar concentration c x of a solution of a solute species X is the number of moles of that species that is contained in 1 liter of the solution not 1 L of the solvent. Calculate the grams of the solute. From mol dm-3 we know we have to calculate the molarity. N number of moles of solute and V the volume of solution The unit of molar concentration is molar symbolized by M which has the dimensions of molL or mol L-1. Some concentrations are expressed in terms the species actually measured eg mgL of NO3- mass of nitrate ions per liter.
 Source: slideplayer.com
Source: slideplayer.com
In chemical notation square brackets around the name or formula of the solute represent the. Moles 5556 mol. Multiply 1 L X the density 154 gmL X 1000 mLL. Number of moles 1000 g 18 g mol-1. Taking into account cell pathlength and compound concentration we can arrive at a Molar circular dichroism Δε.
 Source: pinterest.com
Source: pinterest.com
Milligrams per litre mgL mgL-1 for water samples or mass per mass eg. First we must convert the mass of NaCl in grams into moles. The solvent may be a gas or liquid but is commonly a liquid. 1 g glucose dissolved in water to a final volume of 100 ml solution gives a 1 wv glucose solution. Since Molarity is concentration expressed in moles per liter we just have to divide the 556 mol by 1 L to get 556 M.
 Source: slideplayer.com
Source: slideplayer.com
Latex100 text grams NaCl times fractext1 mole584 text gmole 017 text moles NaCllatex Then we divide the number of moles by the total solution volume to get concentration. As you can see this number is so large that we can dissolve chemicals in. The molar mass of sulfuric acid is 9809 gmol. Specifically molarity is defined as. To calculate molar ellipticity the sample concentration gL cell pathlength cm and the molecular weight gmol must be known.
 Source: slideplayer.com
Source: slideplayer.com
The molar concentration of a solution is the number of moles of solute per litre of solvent molL1. Concentration moles volume. Another definition is that concentration is the ratio of solute in a solution to either solvent or total solution. One mole represents N A entities atoms molecules ions electrons where N A 60210 23 mol -1 is the Avogadro constant. Density mass of a unit volume of a material substance.
 Source: youtube.com
Source: youtube.com
This is a unit specific for. 25M means 25 moles of sulfuric acid per one liter of solution. The solvent may be a gas or liquid but is commonly a liquid. To calculate molar ellipticity the sample concentration gL cell pathlength cm and the molecular weight gmol must be known. First we must convert the mass of NaCl in grams into moles.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
The molar concentration of a solution is the number of moles of solute per litre of solvent molL1. An aqueous solution that contains 1 mol 342 g of sucrose in enough water to give a final volume of 100 L has a sucrose concentration of 100 molL or 100 M. Hence 100 mgLsewater 100 mgL x 1 mL103 g x 1 L1000 mL x 1000 mgg 0971 mgkg or 0971 ppm Note 2. 25M means 25 moles of sulfuric acid per one liter of solution. The conversion factor between the units molz eq val and g-equivalent is one meaning 1 mmolL 1z 1 meqL 1 mvalL 1 мг-эквл 1 mg-eqL.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title what is meant by concentration in unit g mol 1 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






