Your What is free energy change when 1 mole of water images are available in this site. What is free energy change when 1 mole of water are a topic that is being searched for and liked by netizens today. You can Get the What is free energy change when 1 mole of water files here. Get all royalty-free vectors.
If you’re looking for what is free energy change when 1 mole of water images information linked to the what is free energy change when 1 mole of water topic, you have visit the ideal site. Our website frequently provides you with hints for seeing the maximum quality video and image content, please kindly hunt and find more enlightening video articles and graphics that match your interests.
What Is Free Energy Change When 1 Mole Of Water. Asked Nov 16 2019 in Chemical thermodynamics by Ranjeet01 590k points. Calculate the free energy change when 1 mole of NaCl is dissolved in water at 25C. Extra Practice Problems. Answer andor Hint This problem asks you to remember or derive the pressure dependence of the Gibbs Free Energy.
 18 8 Gibbs Energy Changes In Chemical Reactions Chemistry Libretexts From chem.libretexts.org
18 8 Gibbs Energy Changes In Chemical Reactions Chemistry Libretexts From chem.libretexts.org
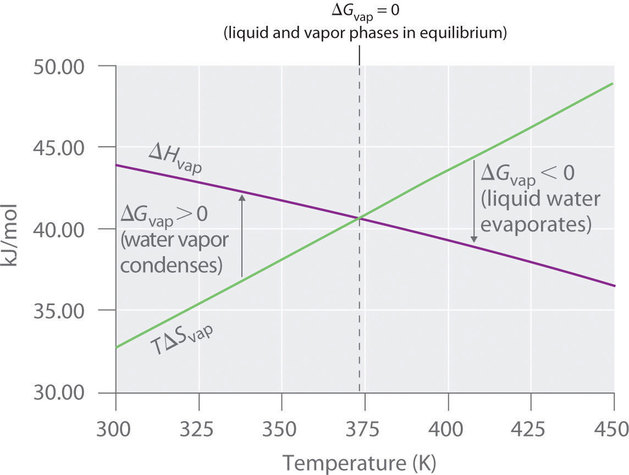
Dec 15 2019 Whats the free power change when 1 mole of water at 100and 1 atm. From Enthalpy Changes and Entropy Changes. The evaporation of one mole of water at 298 K has a standard free energy change of 858 kJ. Hence the total change is Δ G 0 517 c a l that is Δ G 517 c a l. From The Ionization Constant Calculate The Standard Gibbs Free-energy Change For The Ionization. Extra Practice Problems.
593 x 10-6 kj.
At fixed stress w V 2 V 1 pdV. From Free Energies of Formation. The answer here remained 517cal because initially the change in Gibbs free energy when 10 mole of water at 100 C and 1 atm pressure is converted into steam at 100 C and 1 atm pressure is 0 cal. Lattice energy of NaCl 7778 kJ mol -1 Hydration energy -7741 kJ mol -1 and ΔS 0043 kJK -1 mol -1 at 298 K. Answer andor Hint This problem asks you to remember or derive the pressure dependence of the Gibbs Free Energy. What is free energy change G when 1 mole of water at 100C and 1 ATM pressure is converted into steam at 100C and 2 ATM pressureHere at 100c water and g.
 Source: secondaryscience4all.wordpress.com
Source: secondaryscience4all.wordpress.com
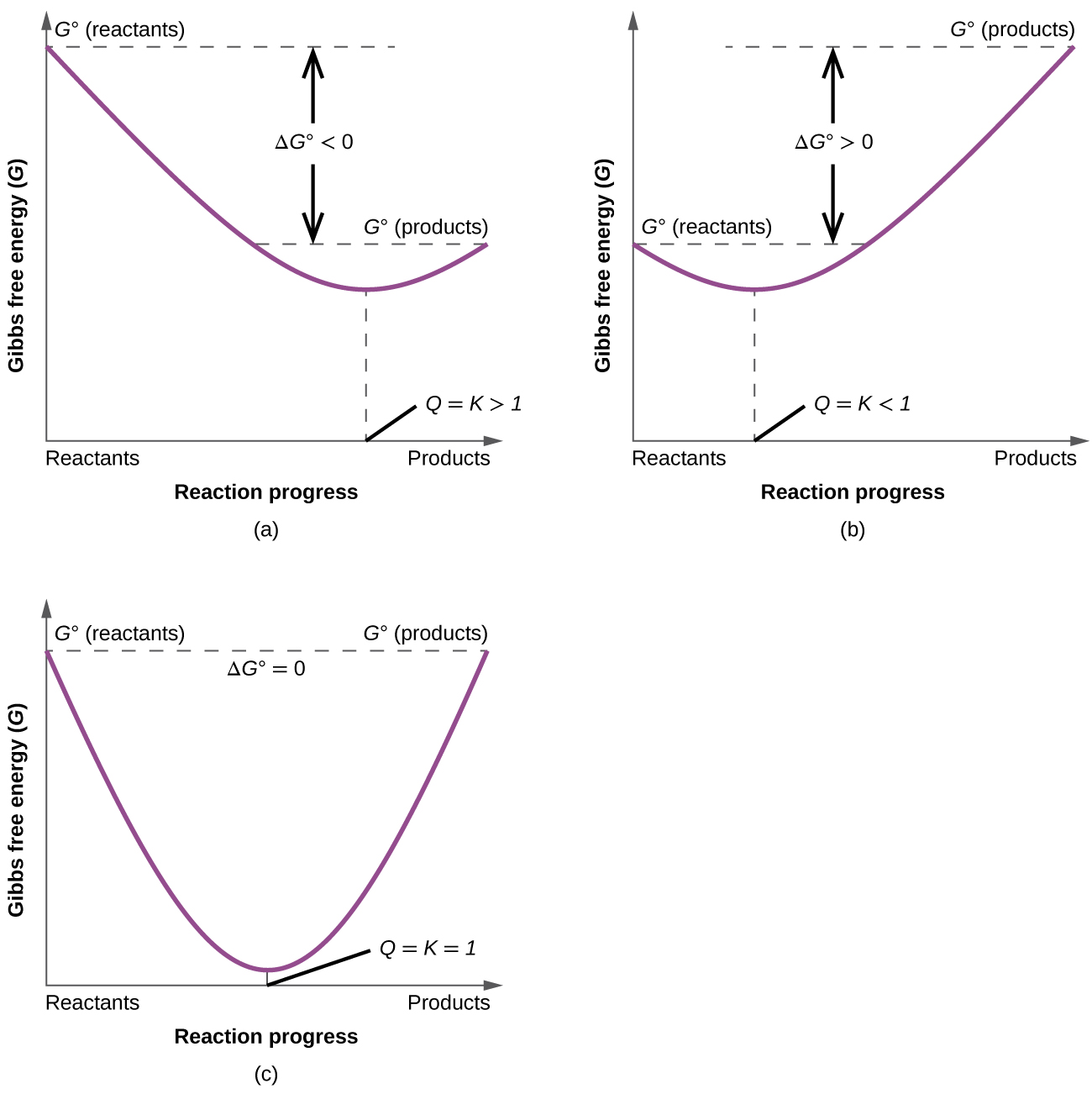
The free energy change of a chemical process under standard state conditions G o can be determined four different ways. Extra Practice Problems. Lattice energy of NaCl 7778 kJ mol -1 Hydration energy -7741 kJ mol -1 and ΔS 0043 kJK -1 mol -1 at 298 K. Heat of vaporization temperature Here heat of vaporisation 540 calgm. Answer andor Hint This problem asks you to remember or derive the pressure dependence of the Gibbs Free Energy.
 Source: slideplayer.com
Source: slideplayer.com
Physics questions and answers. The evaporation of one mole of water at 298 K has a standard free energy change of 858 kJ. The free energy change Δ G when 10 mole of water at 1 0 0 C and 1 atm pressure is converted into steam at 1 0 0 C and 1 atm pressure is 0 cal as the system is at equilibrium. ΔS for dissolution 0043 kJ mol-1and hydration energy of NaCl -7741 kJ mol-1. H 2 O l H 2 O g Δ G 858 kJ H 2 O l H 2 O g Δ G 858 kJ a Is the evaporation of water under standard thermodynamic conditions spontaneous.
 Source: opentextbc.ca
Source: opentextbc.ca
-593 x 10-6 kj. 2 540 cal 3 620 cal 4 Zero. The standard Gibbs free energy change ΔG0is related to equilibrium constant Kp as. H 2 O l H 2 O g Δ G 858 kJ H 2 O l H 2 O g Δ G 858 kJ a Is the evaporation of water under standard thermodynamic conditions spontaneous. Lattice energy of NaCl 7778 kJ mol-1.
 Source: chem.libretexts.org
Source: chem.libretexts.org
What is the free energy change ΔGWhen 10 mole of water at 1000C and 1atm pressure is converted into steam at 1000C and 1atm pressure. -593 x 10-6 kj. Calculate the free energy change when 1 mole of NaCl is dissolved in water at 25C. From The Ionization Constant Calculate The Standard Gibbs Free-energy Change For The Ionization Of Acetic Acid In Water At 25C. What is free energy change G when 1 mole of water at 100C and 1 ATM pressure is converted into steam at 100C and 2 ATM pressureHere at 100c water and g.
 Source: researchgate.net
Source: researchgate.net
What is the free energy change DeltaG When 10 mole of water at 100C and 1 atm pressure is converted into steam at 100C and 1 atm pressure. What is the free energy change ΔGWhen 10 mole of water at 1000C and 1atm pressure is converted into steam at 1000C and 1atm pressure. Lattice energy of NaCl 7778 kJ mol-1. At fixed stress w V 2 V 1 pdV. What is the free energy change DeltaG When 10 mole of water at 100C and 1 atm pressure is converted into steam at 100C and 1 atm pressure.
 Source: qsstudy.com
Source: qsstudy.com
The answer here remained 517cal because initially the change in Gibbs free energy when 10 mole of water at 100 C and 1 atm pressure is converted into steam at 100 C and 1 atm pressure is 0 cal. The free energy change of a chemical process under standard state conditions G o can be determined four different ways. Answer andor Hint This problem asks you to remember or derive the pressure dependence of the Gibbs Free Energy. 20oC and 1 atm pressure. F is 96485 kJ volt-1 mole-1 the Faraday.
 Source: book.bionumbers.org
Source: book.bionumbers.org
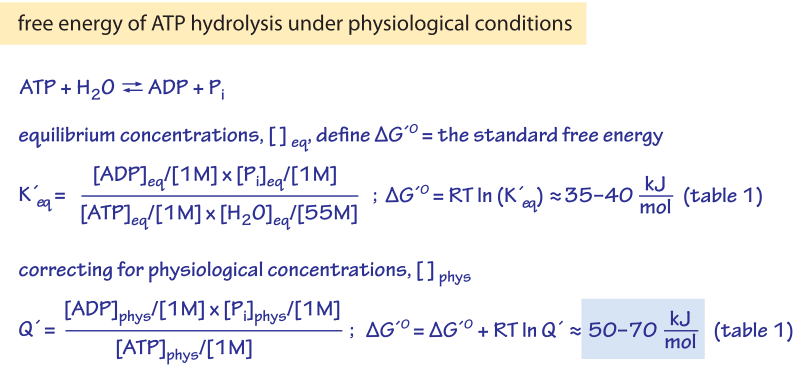
Heat of vaporization temperature Here heat of vaporisation 540 calgm. Calculate the free energy change when 1 mole of NaCl is dissolved in water at 298 K. From Enthalpy Changes and Entropy Changes. The evaporation of one mole of water at 298 K has a standard free energy change of 858 kJ. What would be the free energy change involved in transporting 106 mole of glucose from the medium into the cell if the intracellular and extracellular concentrations were 1 mM and 10 mM respectively.
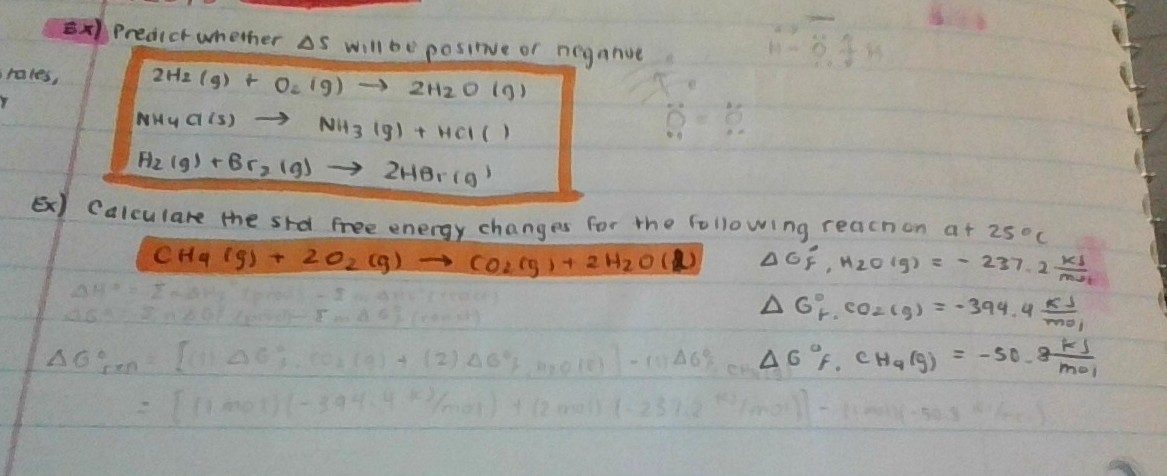 Source: socratic.org
Source: socratic.org
What would be the free energy change involved in transporting 106 mole of glucose from the medium into the cell if the intracellular and extracellular concentrations were 1 mM and 10 mM respectively. What is the free energy change G when 10 mole of water at 100⁰C and 1 atm pressure is converted into steam. Given lattice energy of NaCl 7741 kj mol and Δ S 0043 Kj K 1mol 1 at 298 K. What is the free energy change G when 10 mole of water at 100⁰C and 1 atm pressure is converted into steam at 100⁰C and 2 atm pressure. DE TdS pdV.
 Source: ppt-online.org
Source: ppt-online.org
Calculate the free energy change when 1 mole of NaCl is dissolved in water at 25C. Evaluating Relative Molar Entropy for Chemicals Calculatingp1 ΔGfor Reactions Math p5 Evaluating ΔS for Reactions non-math p2 ΔG ΔH ΔS Equilibrium and Temperature p6 Calculating p2ΔS for Reactions Math Answers p7. EditedOct 9 2018by Tannu. ΔS for dissolution 0043 kJ mol-1and hydration energy of NaCl -7741 kJ mol-1. Lattice energy of NaCl 7778 kJ mol -1 Hydration energy -7741 kJ mol -1 and ΔS 0043 kJK -1 mol -1 at 298 K.
 Source: toppr.com
Source: toppr.com
The standard Gibbs free energy change ΔG0is related to equilibrium constant Kp as. The evaporation of one mole of water at 298 K has a standard free energy change of 858 kJ. Calculate the free energy change when 1 mole of NaCl is dissolved in water at 25C. Calculate the free energy change when 1 mole of NaCl is dissolved in water at 298 K. The usual free power of formation is the free power change that accompanies the formation of 1 mole of a substance from its components of their customary states.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
From The Ionization Constant Calculate The Standard Gibbs Free-energy Change For The Ionization Of Acetic Acid In Water At 25C. This tells us that when 1 mole of liquid water at 2982 K and atmospheric pressure is formed from its elements in their standard states that is from hydrogen gas H 2g and oxygen gas O 2g the change in standard Gibbs free energy is 2372 kJ and the reaction is spontaneous because the sign of ΔG is negative. What is the free energy change ΔG when 10 mole of water at 100ºC and 1 atm pressure is converted into steam. From The Ionization Constant Calculate The Standard Gibbs Free-energy Change For The Ionization. The free energy change of a chemical process under standard state conditions G o can be determined four different ways.

What is the free energy change when 1 mole of water at 100 degree celsius and 1 atm pressure is converted into steam at 100 degree celsius and to 2 atm - 2604914. EditedOct 9 2018by Tannu. F is 96485 kJ volt-1 mole-1 the Faraday. Here is a micro derivation. The entropy change.
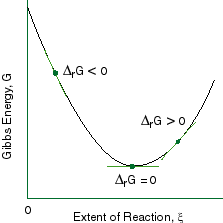 Source: public.asu.edu
Source: public.asu.edu
Assuming the volume to be constant find G Change in Gibbs Free Energy for compression of 1 mole of the liquid from 1 to 100 atm in caloriesmole. What is free energy change G when 1 mole of water at 100C and 1 ATM pressure is converted into steam at 100C and 2 ATM pressureHere at 100c water and g. From Free Energies of Formation. Requested Nov 16 2019 in Chemical thermodynamics by Ranjeet01 590k factors. Calculate the free energy change when 1 mole of NaCl is dissolved in water at 298 K.
 Source: clutchprep.com
Source: clutchprep.com
Physics questions and answers. The answer here remained 517cal because initially the change in Gibbs free energy when 10 mole of water at 100 C and 1 atm pressure is converted into steam at 100 C and 1 atm pressure is 0 cal. From The Ionization Constant Calculate The Standard Gibbs Free-energy Change For The Ionization Of Acetic Acid In Water At 25C. Calculate the free energy change when 1 mole of NaCl is dissolved in water at 298 K. 20oC and 1 atm pressure.
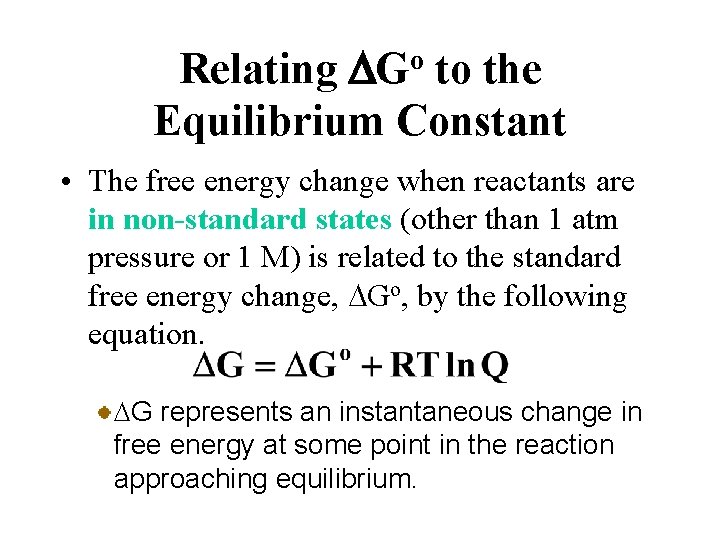 Source: slidetodoc.com
Source: slidetodoc.com
Evaluating Relative Molar Entropy for Chemicals Calculatingp1 ΔGfor Reactions Math p5 Evaluating ΔS for Reactions non-math p2 ΔG ΔH ΔS Equilibrium and Temperature p6 Calculating p2ΔS for Reactions Math Answers p7. Extra Practice Problems. From The Ionization Constant Calculate The Standard Gibbs Free-energy Change For The Ionization. The standard Gibbs free energy change ΔG0is related to equilibrium constant Kp as. 593 x 10-6 kj.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title what is free energy change when 1 mole of water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






