Your What is equivalent to grams per mole images are available. What is equivalent to grams per mole are a topic that is being searched for and liked by netizens today. You can Get the What is equivalent to grams per mole files here. Find and Download all free photos and vectors.
If you’re searching for what is equivalent to grams per mole pictures information related to the what is equivalent to grams per mole topic, you have visit the right blog. Our website always provides you with hints for refferencing the highest quality video and picture content, please kindly surf and find more informative video content and images that match your interests.
What Is Equivalent To Grams Per Mole. Therefore the mole is a unit for that physical quantity. Whereas its equivalent mass would be 133343 444467 geq. This is just like you say dozen is equivalent to 12 a score is 20 and a century is 100. Type in your own numbers in the form to convert the units.
 How To Convert Grams To Moles Very Easy Youtube From youtube.com
How To Convert Grams To Moles Very Easy Youtube From youtube.com
The mole is the SI base unit for substance quantity. Application question 2 What is the molarity of 4g CaCl 2. Thus for example the average mass of a molecule of water is about 180153 daltons and the molar mass of water is about 180153 gmol. A protein whose molecule has an average mass of 64 kDa would have a molar mass of 64 kgmol. Since the unified atomic mass unit symbol. Finally divide the number of grams of the compound by the molar mass of the compound to find the number of moles.
You can always use our grams to moles calculator to check the result.
When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. For example the mass of one mole of Calcium-40 is 3996259098 000000022 grams whereas the mass of one mole of Calcium-42 is 4195861801 000000027 grams and of one mole of Calcium with the normal isotopic mix is 40078 0004 grams. Finding molar mass starts with units of grams per mole gmol. One mole abbreviated mol is equal to 602210 23 molecular entities Avogadros number and each element has a different molar mass depending on the weight of 602210 23 of its atoms 1 mole. Another way of expressing this is that the equivalent weight is defined as the mass in grams of a base that reacts with exactly 1 mole of hydrogen ions H. In this manner how do you convert Milliequivalents to Grams.
 Source: pinterest.com
Source: pinterest.com
Use this page to learn how to convert between moles Oxygen and gram. Thus for example the average mass of a molecule of water is about 180153 daltons and the molar mass of water is about 180153 gmol. Finding molar mass starts with units of grams per mole gmol. One mole abbreviated mol is equal to 602210 23 molecular entities Avogadros number and each element has a different molar mass depending on the weight of 602210 23 of its atoms 1 mole. Moles Units and Conversion Factors Author.
 Source: youtube.com
Source: youtube.com
You can always use our grams to moles calculator to check the result. In other words a mole is a unit that we use to represent 6023 x 10 23 particles of the same matter. U or Da is defined as 112 of the mass of the 12 C atom it follows that the molar mass of a substance measured in grams per mole is numerically equal to its mean atomic or molecular mass measured in Da. 20 molesliter 05L 1mol NaCl 1mol NaCl58 gmol 58 g NaCl. Finding molar mass starts with units of grams per mole gmol.
 Source: wikihow.com
Source: wikihow.com
Therefore the mole is a unit for that physical quantity. Grams to Molar and micro molar conversions. As you already know how the grams to moles conversion work find the number of moles. More information about each measuring unit may be found here. Another property of Avogadros number is that the mass of one mole of a substance is equal to that substances molecular weight.
 Source: in.pinterest.com
Source: in.pinterest.com
Gram equivalent mass is mass of one mole of element molecule ion divided by their valency or number of electrons shared. The SI base unit for amount of substance is the mole. We define a mole as the number equal to Avogadros number. 20 molesliter 05L 1mol NaCl 1mol NaCl58 gmol 58 g NaCl. Whereas its equivalent mass would be 133343 444467 geq.
 Source: in.pinterest.com
Source: in.pinterest.com
Micro molar concentration is a concentration of one one-millionth of a mole micromole per liter typically used in reference to the concentration of a chemical compound in an aqueous solution so you should divide the mass in grams with the molecular weight to have the number of mole. Therefore the mole is a unit for that physical quantity. In other words a mole is a unit that we use to represent 6023 x 10 23 particles of the same matter. Grams to Molar and micro molar conversions. Dalton unit dalton unified atomic mass unit mu.
 Source: youtube.com
Source: youtube.com
The mole is the SI base unit for substance quantity. Note that rounding errors may occur so always check the results. U or Da is defined as 112 of the mass of the 12 C atom it follows that the molar mass of a substance measured in grams per mole is numerically equal to its mean atomic or molecular mass measured in Da. This property simplifies many chemical computations. Type in your own numbers in the form to convert the units.
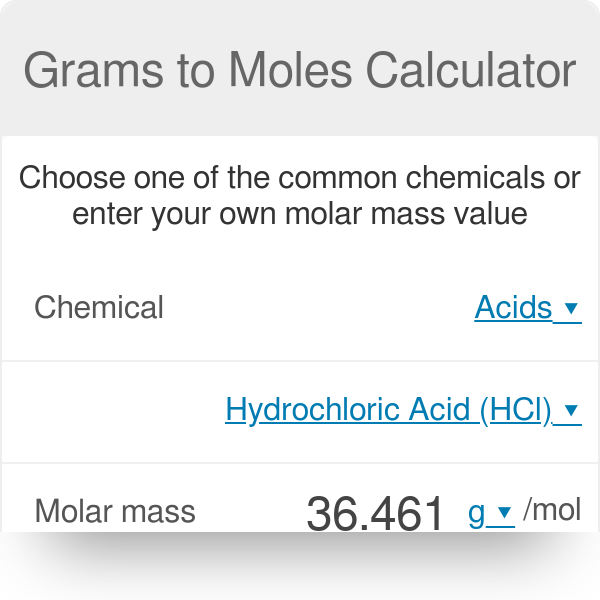 Source: omnicalculator.com
Source: omnicalculator.com
You can always use our grams to moles calculator to check the result. Thus for example the average mass of a molecule of water is about 180153 daltons and the molar mass of water is about 180153 gmol. A protein whose molecule has an average mass of 64 kDa would have a molar mass of 64 kgmol. How many moles are in 7537 grams of sodium chloride NaCl. The molar mass of any element can be determined by finding the atomic mass of the element on the periodic table.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Another way of expressing this is that the equivalent weight is defined as the mass in grams of a base that reacts with exactly 1 mole of hydrogen ions H. This is just like you say dozen is equivalent to 12 a score is 20 and a century is 100. U or Da is defined as 112 of the mass of the 12 C atom it follows that the molar mass of a substance measured in grams per mole is numerically equal to its mean atomic or molecular mass measured in Da. To convert grams to moles start by multiplying the number of atoms by the atomic weight for each element in the compound. Then add all of your answers together to find the molar mass of the compound.
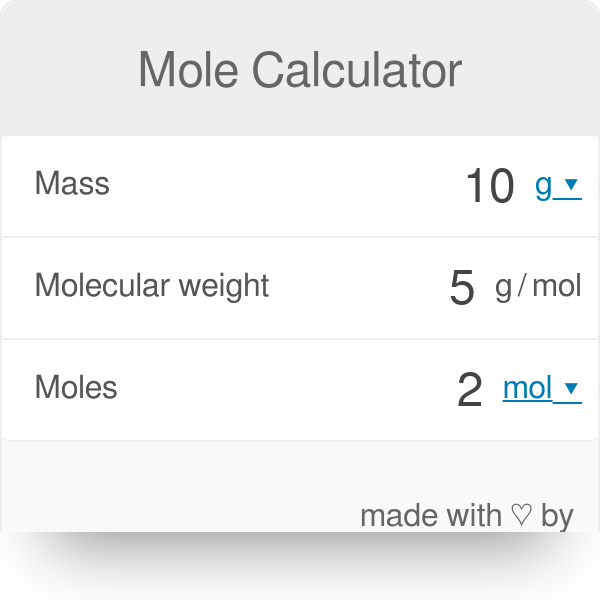 Source: omnicalculator.com
Source: omnicalculator.com
One mole abbreviated mol is equal to 602210 23 molecular entities Avogadros number and each element has a different molar mass depending on the weight of 602210 23 of its atoms 1 mole. Use this page to learn how to convert between moles Oxygen and gram. For example the molar mass of oxygen is 16 grams and one millimole of oxygen contains 0016 grams or 16 milligrams. The mole was defined in such a way that the molar mass of a compound in gmol is numerically equal for all practical purposes to the average mass of one molecule in daltons. This property simplifies many chemical computations.
 Source: youtube.com
Source: youtube.com
This is just like you say dozen is equivalent to 12 a score is 20 and a century is 100. Click to see full answer. One mole abbreviated mol is equal to 602210 23 molecular entities Avogadros number and each element has a different molar mass depending on the weight of 602210 23 of its atoms 1 mole. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. The amu is the unit of mass in the American system of units.
 Source: in.pinterest.com
Source: in.pinterest.com
Another way of expressing this is that the equivalent weight is defined as the mass in grams of a base that reacts with exactly 1 mole of hydrogen ions H. Another property of Avogadros number is that the mass of one mole of a substance is equal to that substances molecular weight. Grams to Moles Conversion Formula Questions. One mEq is equal to 111000 of the gram -equivalent weight GEW of a solute. It is equal to 17th of a gram or 014 grams.
 Source: youtube.com
Source: youtube.com
Gram equivalent mass is mass of one mole of element molecule ion divided by their valency or number of electrons shared. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. This is just like you say dozen is equivalent to 12 a score is 20 and a century is 100. Grams to Molar and micro molar conversions. Since the unified atomic mass unit symbol.
 Source: study.com
Source: study.com
Since the unified atomic mass unit symbol. The number of milligrams per millimole is equivalent to the number of grams per mole. Eg molar mass of AlCl3 is 13334 gmol. U or Da is defined as 112 of the mass of the 12 C atom it follows that the molar mass of a substance measured in grams per mole is numerically equal to its mean atomic or molecular mass measured in Da. Therefore the mole is a unit for that physical quantity.
 Source: pinterest.com
Source: pinterest.com
Click to see full answer. Eg molar mass of AlCl3 is 13334 gmol. The number of milligrams per millimole is equivalent to the number of grams per mole. A protein whose molecule has an average mass of 64 kDa would have a molar mass of 64 kgmol. Therefore the mole is a unit for that physical quantity.
 Source: wikihow.com
Source: wikihow.com
When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. Gram equivalent mass is mass of one mole of element molecule ion divided by their valency or number of electrons shared. This is known as the molar mass M and has the units g mol-1 grams per mole of substance The relationship between molar mass mass and moles can be expressed as a mathematical equation as shown below. Thus for example the average mass of a molecule of water is about 180153 daltons and the molar mass of water is about 180153 gmol. A protein whose molecule has an average mass of 64 kDa would have a molar mass of 64 kgmol.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title what is equivalent to grams per mole by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






