Your What is a dalton in grams per mole images are available in this site. What is a dalton in grams per mole are a topic that is being searched for and liked by netizens today. You can Get the What is a dalton in grams per mole files here. Get all free vectors.
If you’re looking for what is a dalton in grams per mole images information linked to the what is a dalton in grams per mole interest, you have pay a visit to the ideal site. Our website frequently gives you hints for viewing the maximum quality video and picture content, please kindly search and locate more enlightening video articles and images that fit your interests.
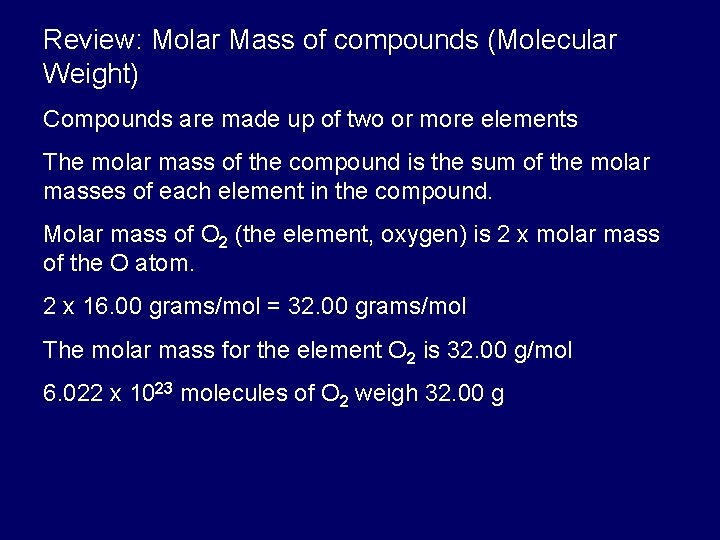
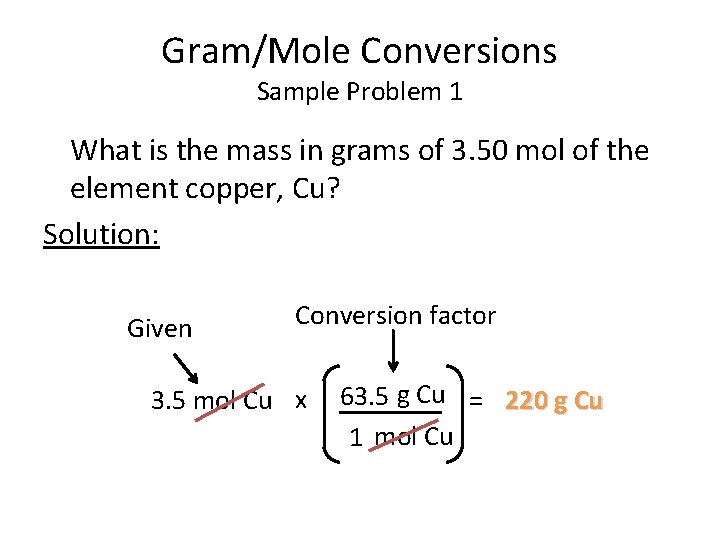
What Is A Dalton In Grams Per Mole. The dalton symbol Da is also sometimes used as a unit of molar mass especially in biochemistry with the definition 1 Da 1 gmol despite the fact that it is strictly a unit of mass 1 Da 1 u 1660 539 066 60 50 10 27 kg as of 2018 CODATA recommended values. One mole contains exactly 6b x 1023 elementary entities. Gram atom is a former term for a mole. Thus a protein with a mass of 64 kDa has a molecular weight of 64000 grams per mole.
 The Mole Ppt Download From slideplayer.com
The Mole Ppt Download From slideplayer.com
What is the numerical value of mole. Begingroup See also this under Molar Mass. The dalton symbol Da is also sometimes used as a unit of molar mass especially in biochemistry with the definition 1 Da 1 gmol despite the fact that it is strictly a unit of mass endgroup. Thus a protein with a mass of 64kDa has a molecular weight of 64000 grams per mole. 1200 g C-12 1 mol C-12 atoms 6022 1023 atoms The number of particles in 1 mole is called Avogadros Number 60221421 x 1023. The SI unit is the dalton.
1 kilogram is equal to 60221366516752E26 dalton or 1000 gram.
The atomic mass of H 1 amu so you could work out the mass of 1 x H atom in grams. The dalton symbol Da is also sometimes used as a unit of molar mass especially in biochemistry with the definition 1 Da 1 gmol despite the fact that it is strictly a unit of mass 1 Da 1 u 166053906660 501027 kg as of 2018 CODATA recommended values. What is the mathematical relationship between atomic mass units amu and grams g. 1g of H atoms 1 mole of H atoms 602 x 1023 atoms. 67 rows How to convert dalton to gram. Dalton Da is an alternate name for the atomic mass unit and kilodalton kDa is 1000 daltons.
 Source: pinterest.com
Source: pinterest.com
1 gram. It is defined as one-twelfth of the mass of a neutral atom of carbon-12 in its ground state. How many kg is Dalton. 1 Da 16605310²⁷ kg. 1 gram.
 Source: slideplayer.com
Source: slideplayer.com
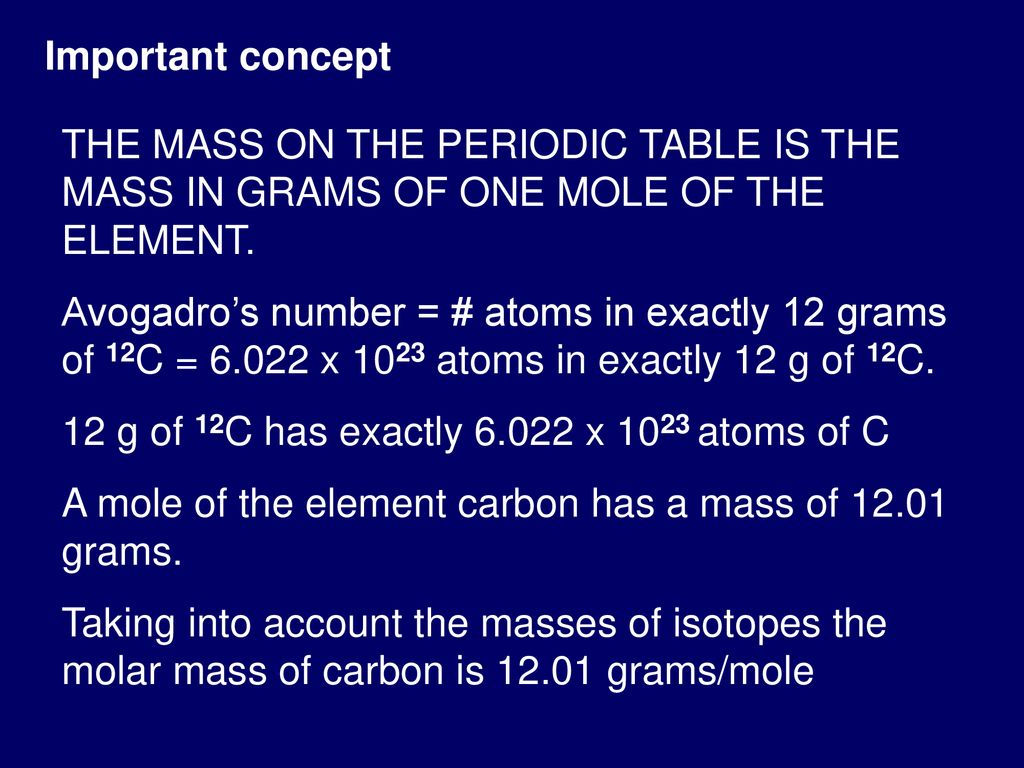
The dalton symbol Da is also sometimes used as a unit of molar mass especially in biochemistry with the definition 1 Da 1 gmol despite the fact that it is strictly a unit of mass 1 Da 1 u 166053906660 501027 kg as of 2018 CODATA recommended values. The atomic mass unit or amu is the mass of one atom of a particular isotope a more general synonym for isotope is nuclide. What is the mathematical relationship between atomic mass units amu and grams g. Note that rounding errors may occur so always check the results. 1200 g C-12 1 mol C-12 atoms 6022 1023 atoms The number of particles in 1 mole is called Avogadros Number 60221421 x 1023.
 Source: slideplayer.com
Source: slideplayer.com
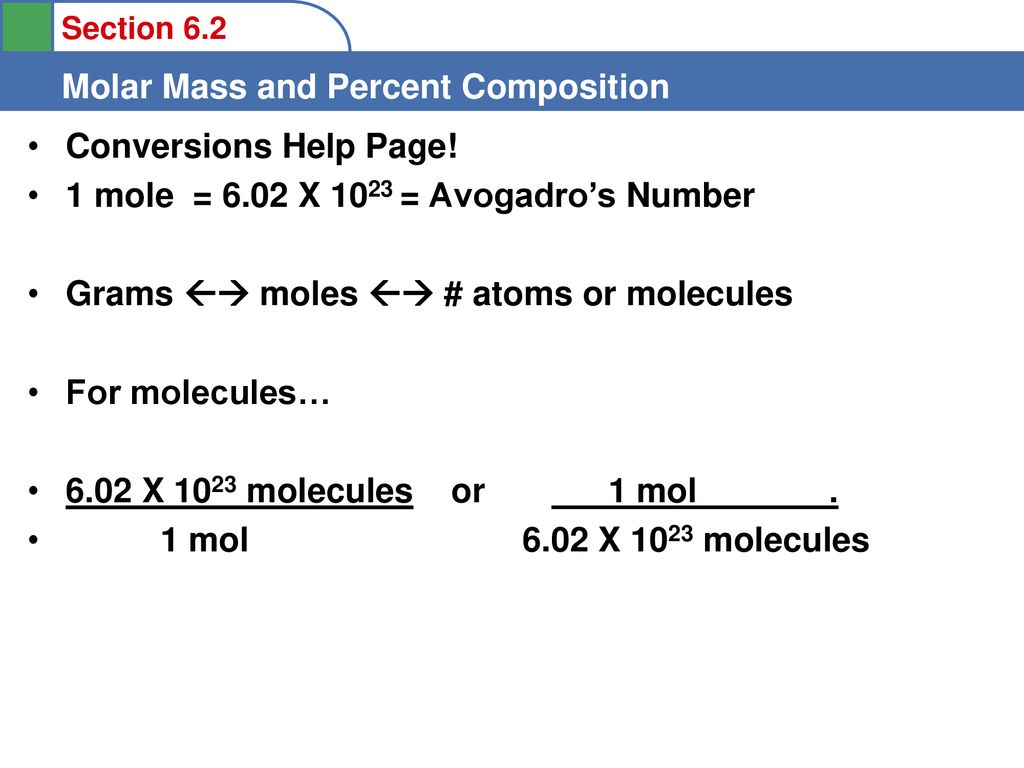
Amu To Grams Conversion Formula Convert Dalton to Other Weight and Mass Units. We assume you are converting between dalton and gram. Amu To Grams Conversion Formula Convert Dalton to Other Weight and Mass Units. Why is the Dalton unit different from the g per mol unit since both of them are the units for atomic weight Here we go again with another question about why something is true when the something is actually not true. 1200 g C-12 1 mol C-12 atoms 6022 1023 atoms The number of particles in 1 mole is called Avogadros Number 60221421 x 1023.
 Source: slideplayer.com
Source: slideplayer.com
Note that rounding errors may occur so always check the results. The French physicist Jean Perrin called the number of units in the sum of one mole Avogadro a few years later. One Mole is the molecular weight expressed as grams - so a mole of Carbon 12 is BY DEFINITION exactly 12 grams. The value of the mole is equal to the number of atoms in exactly 12 grams of pure carbon-12. The dalton symbol Da is also sometimes used as a unit of molar mass especially in biochemistry with the definition 1 Da 1 gmol despite the fact that it is strictly a unit of mass 1 Da 1 u 1660 539 066 60 50 10 27 kg as of 2018 CODATA recommended values.
 Source: slidetodoc.com
Source: slidetodoc.com
Thus a protein with a mass of 64kDa has a molecular weight of 64000 grams per mole. Why is the Dalton unit different from the g per mol unit since both of them are the units for atomic weight Here we go again with another question about why something is true when the something is actually not true. We couldnt find a conversion between dalton and gmole Do a quick conversion. The amu is the same number as. The dalton symbol Da is also sometimes used as a unit of molar mass especially in biochemistry with the definition 1 Da 1 gmol despite the fact that it is strictly a unit of mass 1 Da 1 u 166053906660 501027 kg as of 2018 CODATA recommended values.
 Source: pinterest.com
Source: pinterest.com
The atomic mass of H 1 amu so you could work out the mass of 1 x H atom in grams. What is the numerical value of mole. The dalton noting that. Thus a protein with a mass of 64kDa has a molecular weight of 64000 grams per mole. The French physicist Jean Perrin called the number of units in the sum of one mole Avogadro a few years later.
 Source: slidetodoc.com
Source: slidetodoc.com
Amu To Grams Per Mole. Gram atom is a former term for a mole. Thus a protein with a mass of 64kDa has a molecular weight of 64000 grams per mole. It is defined as one-twelfth of the mass of a neutral atom of carbon-12 in its ground state. Thus a protein with a mass of 64kDa has a molecular weight of 64000 grams per mole.
 Source: pinterest.com
Source: pinterest.com
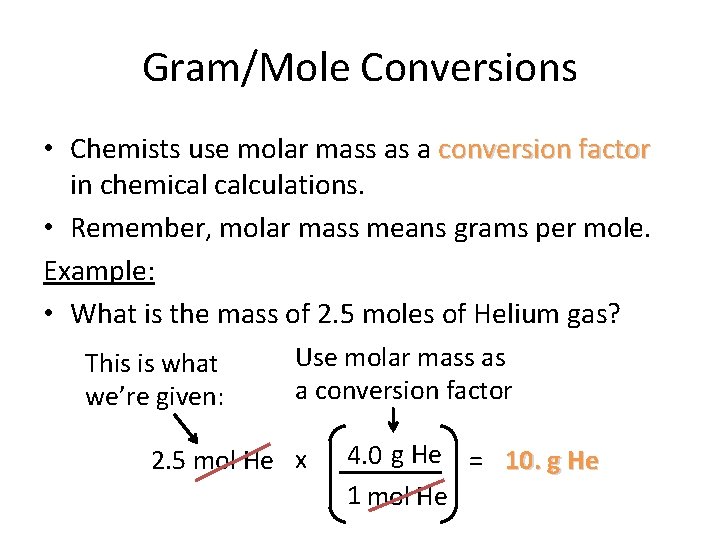
The French physicist Jean Perrin called the number of units in the sum of one mole Avogadro a few years later. The SI base unit for mass is the kilogram. 1g of H atoms 1 mole of H atoms 602 x 1023 atoms. Mass is dalton per entityand because of the mole definition as an Avogadro number of entities dalton per entity is exactly equal to the macroscopic units gram per mole or kilogram per kilomole. Answer 1 of 2.
 Source: studyres.com
Source: studyres.com
Converting Daltons to gmol One Dalton is one AMU atomic Mass Unit. One AMU is 112th of the weight of a Carbon 12 Atom. Converting Daltons to gmol One Dalton is one AMU atomic Mass Unit. How many kg is Dalton. The French physicist Jean Perrin called the number of units in the sum of one mole Avogadro a few years later.
 Source: slidetodoc.com
Source: slidetodoc.com
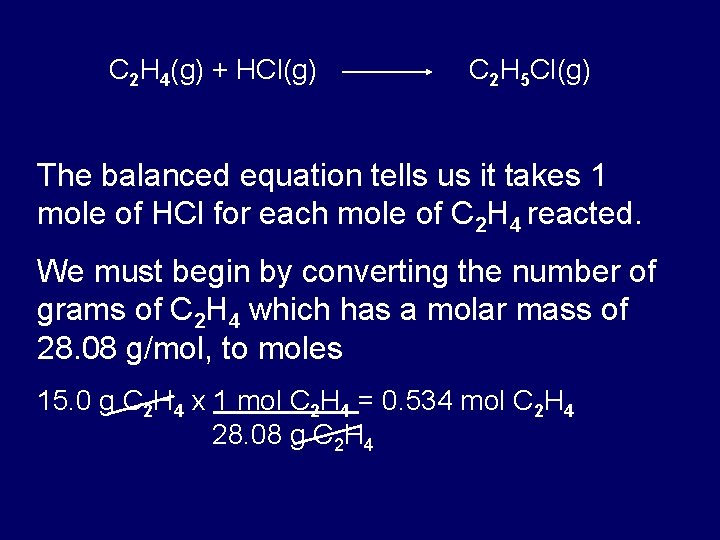
What is the mathematical relationship between atomic mass units amu and grams g. The value of the mole is equal to the number of atoms in exactly 12 grams of pure carbon-12. Thus a protein with a mass of 64 kDa has a molecular weight of 64000 grams per mole. 1g of H atoms 1 mole of H atoms 602 x 1023 atoms. 1 daltons 16605402E-27 gmole using the online calculator for metric conversions.
 Source: pinterest.com
Source: pinterest.com
Convert 15 dalton to g. The SI unit is the dalton. One mole contains exactly 6b x 1023 elementary entities. It is defined as 112th the mass of one carbon-12 atom. Answer 1 of 2.
 Source: slideplayer.com
Source: slideplayer.com
One AMU is 112th of the weight of a Carbon 12 Atom. Why is the Dalton unit different from the g per mol unit since both of them are the units for atomic weight Here we go again with another question about why something is true when the something is actually not true. 67 rows How to convert dalton to gram. Convert 15 dalton to g. The value of the mole is equal to the number of atoms in exactly 12 grams of pure carbon-12.
 Source: slidetodoc.com
Source: slidetodoc.com
We assume you are converting between dalton and gram. The value of the mole is equal to the number of atoms in exactly 12 grams of pure carbon-12. The SI base unit for mass is the kilogram. Thus a protein with a mass of 64kDa has a molecular weight of 64000 grams per mole. Amu To Grams Conversion Formula Convert Dalton to Other Weight and Mass Units.
 Source: slideplayer.com
Source: slideplayer.com
The value of the mole is equal to the number of atoms in exactly 12 grams of pure carbon-12. The atomic mass unit or amu is the mass of one atom of a particular isotope a more general synonym for isotope is nuclide. The dalton symbol Da is also sometimes used as a unit of molar mass especially in biochemistry with the definition 1 Da 1 gmol despite the fact that it is strictly a unit of mass 1 Da 1 u 1660 539 066 60 50 10 27 kg as of 2018 CODATA recommended values. Thus a protein with a mass of 64 kDa has a molecular weight of 64000 grams per mole. The atomic mass of H 1 amu so you could work out the mass of 1 x H atom in grams.
 Source: pinterest.com
Source: pinterest.com
Thus a protein with a mass of 64kDa has a molecular weight of 64000 grams per mole. What is the mathematical relationship between atomic mass units amu and grams g. The atomic mass of H 1 amu so you could work out the mass of 1 x H atom in grams. One Mole is the molecular weight expressed as grams - so a mole of Carbon 12 is BY DEFINITION exactly 12 grams. Thus a protein with a mass of 64kDa has a molecular weight of 64000 grams per mole.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title what is a dalton in grams per mole by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






