Your What is 1 mole of sodium hydroxide images are available in this site. What is 1 mole of sodium hydroxide are a topic that is being searched for and liked by netizens today. You can Download the What is 1 mole of sodium hydroxide files here. Download all royalty-free vectors.
If you’re looking for what is 1 mole of sodium hydroxide images information linked to the what is 1 mole of sodium hydroxide keyword, you have visit the right blog. Our site always provides you with hints for seeing the maximum quality video and image content, please kindly surf and find more informative video articles and graphics that match your interests.
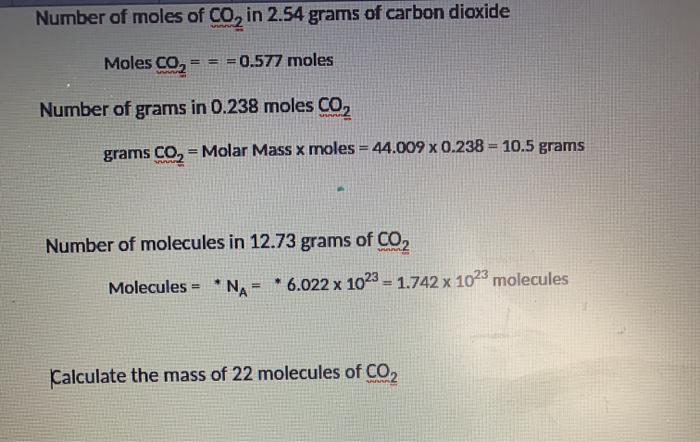
What Is 1 Mole Of Sodium Hydroxide. Add all the molar masses of what composes sodium hydroxide NaOH Na x 1 22989 gmol O x 1 15999 gmol H x 1 1008 gmol. Sodium Hydroxide molecular weight. Avogadro constant- So molecules of NaOH are present in 1 mole of NaOH. We can use this information to make predictions about the amount of products and reactants in the reaction.
 Strong Acids And Bases Mcat Chemistry Cheat Sheet Study Guide Studypk Chemistry Lessons Chemistry Education Chemistry Classroom From pinterest.com
Strong Acids And Bases Mcat Chemistry Cheat Sheet Study Guide Studypk Chemistry Lessons Chemistry Education Chemistry Classroom From pinterest.com
Given Molar mass of NaOH 400 gmol. Sodium hydroxide weighs 213 gram per cubic centimeter or 2 130 kilogram per cubic meter ie. For example if there are 50 moles of NaOH in 500 liters of solution it is a 01 molar. So concetration004400001 N So pOH3 pH143110 We can also do the problem in a. This is a table of density kgL and the corresponding concentration weight of Sodium Hydroxide in water at a temperature of 20 degrees centigrade. Total molar mass of sodium hydroxide is 39996 gmol.
The molarity of NaOH shows the concentration of sodium hydroxide in a solution in terms of moles per liter.
What is the molar mass of sodium hydroxide. Mole is a widely used unit for calculating the amount of matter of a substance. The pH of the solution. Add sodium hydroxide to water do not add water to solid sodium hydroxide. Weight of NaOH in 10 liters 0410004 Now weigh of NaOH in liter is 004 g. The molecular formula of sodium hydroxide is NaOH.
 Source: pinterest.com
Source: pinterest.com
The molecular formula for Sodium Hydroxide is NaOH. One moles of sodium hydroxide 40 gm of sodium hydroxide. MO 15999 gmol. Answer 1 of 6. What is the molar mass of sodium hydroxide.
 Source: pinterest.com
Source: pinterest.com
Note that rounding errors may occur so always check the results. The SI base unit for amount of substance is the mole. It is a chemical base and comes packaged as a white solid. 1 mole is equal to 1 moles Sodium Hydroxide or 3999711 grams. 1 mole is equal to 1 moles Sodium Hydroxide or 3999711 grams.
 Source: pinterest.com
Source: pinterest.com
The level of corrosion depends on the period of contact with the tissue and on the concentration of sodium hydroxide. MH 10079 gmol. 22989770 159994 100794 Percent composition by element. The pH of the solution. Sodium hydroxide is NaOH.
 Source: pinterest.com
Source: pinterest.com
Weight of NaOH in 10 liters 0410004 Now weigh of NaOH in liter is 004 g. Each mole of sodium hydroxide dissolves to give a mole of hydroxide ions in solution. Answer 1 of 6. Note that rounding errors may occur so always check the results. What is 1 N NaOH.
 Source: pinterest.com
Source: pinterest.com
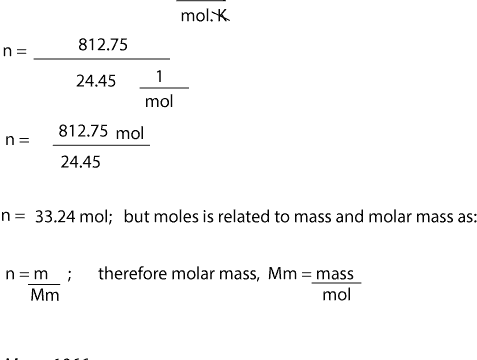
At 25C 77F or 29815K at standard atmospheric pressure. It is named after scientist Amedeo Avogadro and is denoted by. Molecular weight 40 gmol Weight of NaOH is 40586 2344gm Weight of solution volume of solution 1000 mL Density of solution 129 gmL1000 mL 1290 g Weight of solvent water 1290-2344 10556 g 1055618 5864 Mole fraction of solute NaOH number of moles number of moles number of moles of solvent 586 5865864 0090. Now moving back to the question we have been given a sample of 160 grams of Sodium hydroxide. The table was taken from the chemical engineering handbook.
 Source: pinterest.com
Source: pinterest.com
To make 1 N solution dissolve 4000 g of sodium hydroxide in water to make volume 1 liter. Note that rounding errors may occur so always check the results. So concetration004400001 N So pOH3 pH143110 We can also do the problem in a. 7 Is lye sodium hydroxide. What is 1 N NaOH.
 Source: pinterest.com
Source: pinterest.com
MH 10079 gmol. The molecular formula for Sodium Hydroxide is NaOH. For instance 1 mole of Sodium hydroxide is the same as 4000 grams of Sodium hydroxide NaOH molecular weight. Weight of 001 mole of NaOH Strength X Molecular weight 001 x 40 04gm. Stir the sodium hydroxide a little at a time into a large volume of water and then dilute the solution to make one liter.
 Source: pinterest.com
Source: pinterest.com
Given Molar mass of NaOH 400 gmol. Some common bases as sodium hydroxide ammonia and more Base Normality pH Sodium hydroxide 01 N 130 Sodium hydroxide 001 N 120 Sodium metasilicate 01 N 126 Sodium sesquicarbonate 01 N 101 What is the pH of a 002 M sodium hydroxide solution. Molecular weight of Sodium Hydroxide or grams. Sodium hydroxide is NaOH. The molecular formula of sodium hydroxide is NaOH.
 Source: pinterest.com
Source: pinterest.com
Concentrationamount of solutevolume of solution And here this quotient gives1mol0250L40molL-1. The molecular formula of sodium hydroxide is NaOH. Stir the sodium hydroxide a little at a time into a large volume of water and then dilute the solution to make one liter. Answer 1 of 6. Note that rounding errors may occur so always check the results.
 Source: pinterest.com
Source: pinterest.com
1 mole is equal to 1 moles Sodium Hydroxide or 3999711 grams. So we can said. So the equivalent weight of NaOH is 40. It is a chemical base and comes packaged as a white solid. Stir the sodium hydroxide a little at a time into a large volume of water and then dilute the solution to make one liter.
 Source: in.pinterest.com
Source: in.pinterest.com
Molecular weight 40 gmol Weight of NaOH is 40586 2344gm Weight of solution volume of solution 1000 mL Density of solution 129 gmL1000 mL 1290 g Weight of solvent water 1290-2344 10556 g 1055618 5864 Mole fraction of solute NaOH number of moles number of moles number of moles of solvent 586 5865864 0090. Sodium hydroxide is NaOH. This means mass of 1 mole of NaOH 40 g. At 25C 77F or 29815K at standard atmospheric pressure. Each mole of sodium hydroxide dissolves to give a mole of hydroxide ions in solution.
 Source: pinterest.com
Source: pinterest.com
Use this page to learn how to convert between moles Sodium Hydroxide and gram. Avogadro constant- So molecules of NaOH are present in 1 mole of NaOH. The molarity of a sodium hydroxide solution can be determined by dividing the amount of sodium hydroxide in moles present by the number of liters of the overall solution. Two moles of sodium hydroxide on the other hand produces one mole of sodium sulfate 21 ratio and 2 moles of water11 ratio. Total molar mass of sodium hydroxide is 39996 gmol.
 Source: pinterest.com
Source: pinterest.com
If want prepare 1 molar NaOH solution then we need 40 gm NaOH. One moles of sodium hydroxide 40 gm of sodium hydroxide. If want prepare 1 molar NaOH solution then we need 40 gm NaOH. Total molar mass of sodium hydroxide is 39996 gmol. Given Molar mass of NaOH 400 gmol.
 Source: pinterest.com
Source: pinterest.com
Note that rounding errors may occur so always check the results. The molecular formula of sodium hydroxide is NaOH. What is the pH of sodium hydroxide. For instance 1 mole of Sodium hydroxide is the same as 4000 grams of Sodium hydroxide NaOH molecular weight. Some common bases as sodium hydroxide ammonia and more Base Normality pH Sodium hydroxide 01 N 130 Sodium hydroxide 001 N 120 Sodium metasilicate 01 N 126 Sodium sesquicarbonate 01 N 101 What is the pH of a 002 M sodium hydroxide solution.
 Source: pinterest.com
Source: pinterest.com
We can use this information to make predictions about the amount of products and reactants in the reaction. So we can said. The level of corrosion depends on the period of contact with the tissue and on the concentration of sodium hydroxide. Total molar mass of sodium hydroxide is 39996 gmol. MO 15999 gmol.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title what is 1 mole of sodium hydroxide by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.