Your What is 05 moles in grams images are ready. What is 05 moles in grams are a topic that is being searched for and liked by netizens today. You can Find and Download the What is 05 moles in grams files here. Download all royalty-free images.
If you’re searching for what is 05 moles in grams pictures information related to the what is 05 moles in grams topic, you have visit the right blog. Our website always provides you with hints for viewing the highest quality video and picture content, please kindly search and locate more enlightening video content and images that match your interests.
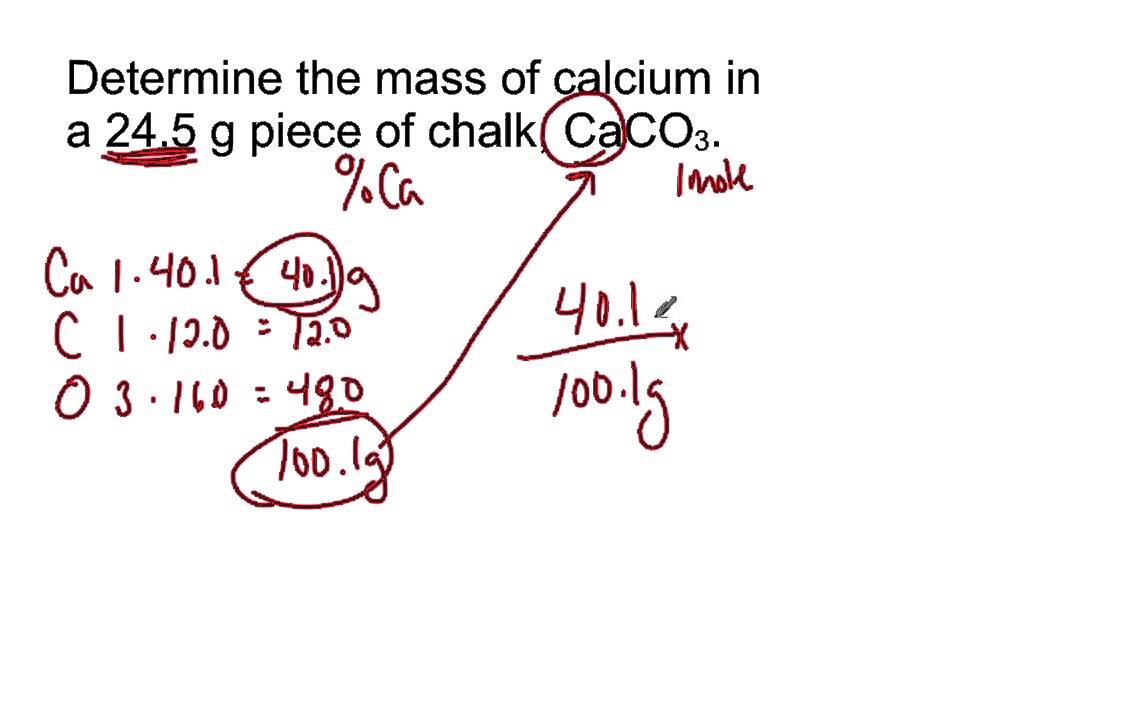
What Is 05 Moles In Grams. The answer is 00087094358027487. C One mole of a monatomic element has a mass equal to its atomic mass expressed in grams. Use this page to learn how to convert between moles H2SO4 and gram. What volume of carbon dioxide at 1000 atm.
 Stoichiometry Notes Equations To Memorize 1 Mol Molar Mass G Get It From The Atomic Mass Add It Up If You Have A Molecule O 15 999 So O 2 31 998 Ppt Download From slideplayer.com
Stoichiometry Notes Equations To Memorize 1 Mol Molar Mass G Get It From The Atomic Mass Add It Up If You Have A Molecule O 15 999 So O 2 31 998 Ppt Download From slideplayer.com
Note that rounding errors may occur so always check the results. Mass in grams of one mole of any element numerically equal to its atomic weight Molar mass of molecules can be determined from the chemical formula and molar masses of elements Each H 2O molecule contains 2 H atoms and 1 O atom Each mole of H 2O molecules contains 2 moles of H and 1 mole of O One mole of O atoms corresponds to 159994 g. In the above problem 5844 gramsmol is the molar mass of NaCl. As you already know how the grams to moles conversion work find the number of moles. E none of the above. The answer is 0058718113128665.
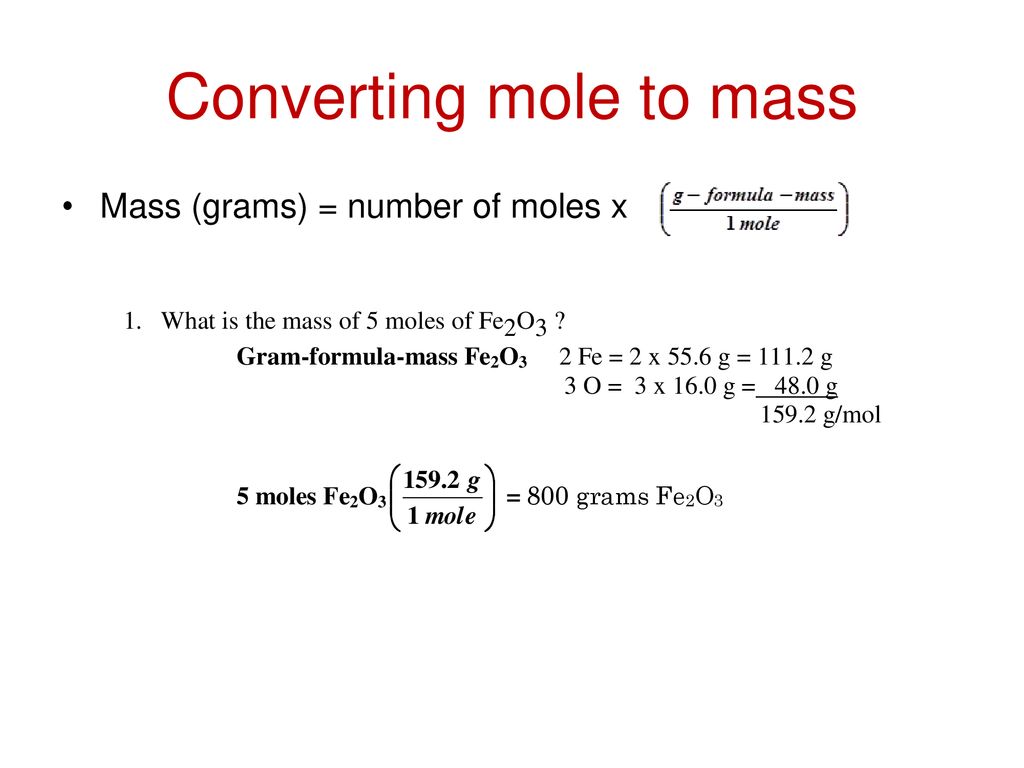
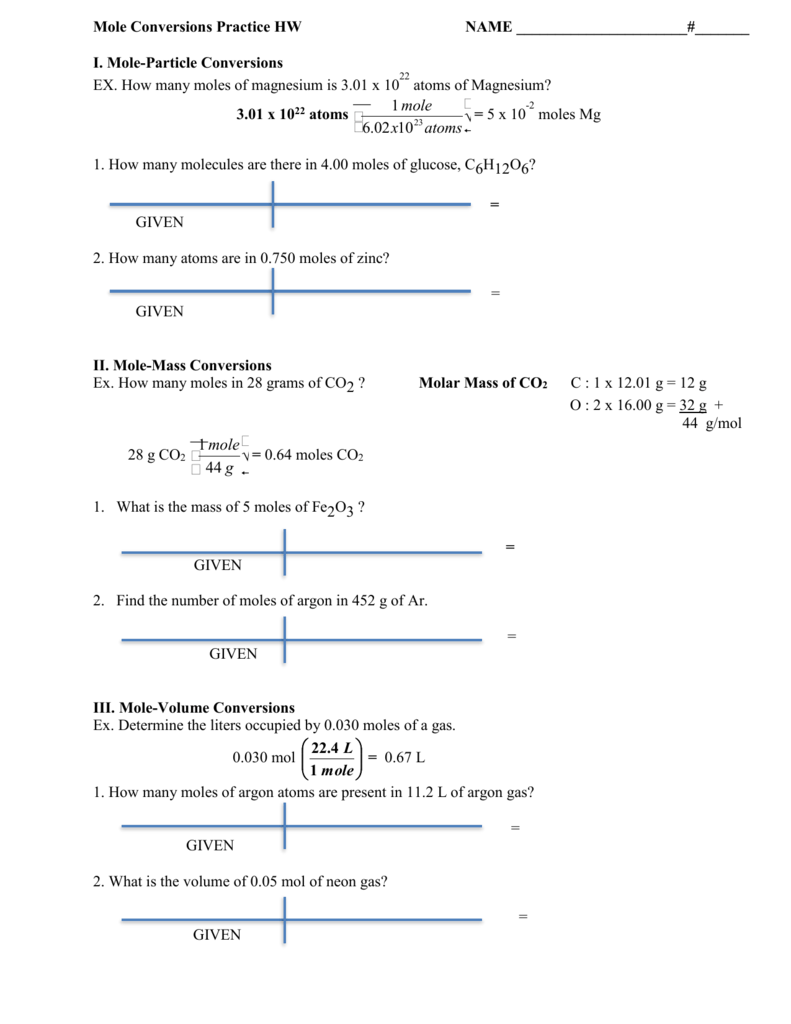
The mole of a substance is related to its mass and molar mass according to the following equation.
21008 3206 418 106076. E none of the above. 6 moles NO2 to grams 276033 grams. The molecular formula for Magnesium is. Molecular weight of Water or grams The molecular formula for Water is H2O. We assume you are converting between moles NH3 and gram.
 Source: slidetodoc.com
Source: slidetodoc.com
You can view more details on each measurement unit. The answer is 00087094358027487. The mass of 005 mole of gold is 985 g. How many grams are in a mole of CH3OH. Multiply the moles given by the substances molar mass.
 Source: youtube.com
Source: youtube.com
1 mole is equal to 1 moles. With the above formula we can obtain the mass of gold. Divide moles by kg of solvent to get molality. 3 moles NO2 to grams 1380165 grams. Molecular weight of NH3 or grams.
 Source: study.com
Source: study.com
Note that rounding errors may occur so always check the results. Where is the molar mass of the substance. B A mole of a monatomic element corresponds to one Avogadros number of atoms. Knowing how to convert grams to moles may be helpful in numerous chemical tasks eg finding the mole fraction of a solution. Molecular weight of In or grams.
 Source: slideplayer.com
Source: slideplayer.com
The answer is 0055508435061792. How many grams are in a mole of CH3OH. The SI base unit for amount of substance is the mole. D One mole of water contains 12 mole of oxygen atoms. Molar mass of gold 19697 gmol.
 Source: slideplayer.com
Source: slideplayer.com
Convert moles to grams and grams to moles. What volume of carbon dioxide at 1000 atm. 0700 mole x 340146 gramsmole 238 grams The answer of 238 g has been rounded to three significant figures because the 0700 value had the least number of significant figures in the problem. E none of the above. Formula mass of acetic acid is 6005 amu moles grams formula mass moles 252 g acetic acid 6005 amu per mole of acetic acid moles 0420 moles Molarity M mol solute 1 liter of solution M 0420 moles acetic acid 0500 liter vinegar solution M 0839 molesL 0839 M.
 Source: slideplayer.com
Source: slideplayer.com
Formula mass of acetic acid is 6005 amu moles grams formula mass moles 252 g acetic acid 6005 amu per mole of acetic acid moles 0420 moles Molarity M mol solute 1 liter of solution M 0420 moles acetic acid 0500 liter vinegar solution M 0839 molesL 0839 M. D One mole of water contains 12 mole of oxygen atoms. The mass of 005 mole of gold is 985 g. Mole mass molar mass. What volume of carbon dioxide at 1000 atm.
 Source: studylib.net
Source: studylib.net
More commonly written for this application as. You can view more details on each measurement unit. C One mole of a monatomic element has a mass equal to its atomic mass expressed in grams. We assume you are converting between moles CH3OH and gram. For an experiment you need to dissolve 005 mole of NaCl in one liter of water.
 Source: slideplayer.com
Source: slideplayer.com
A One mole of atoms makes up an amount of atoms that can be seen with the naked eye. Example 1 Calculate the mass in grams of 36 mol of H 2 SO 4. N 5988 g 18015 gmol 3324 mol. Convert grams to moles. You can view more details on each measurement unit.
 Source: studylib.net
Source: studylib.net
If you know the quantity of mole it can be converted into grams and vice versa. How much NaCl must you weigh out. Discover the similarity between the estimate and the precise outcome. The SI base unit for amount of substance is the mole. 21008 3206 418 106076.
 Source: slideplayer.com
Source: slideplayer.com
We assume you are converting between moles In and gram. E none of the above. We assume you are converting between moles Magnesium and gram. Discover the similarity between the estimate and the precise outcome. You can view more details on each measurement unit.
 Source: knowinsiders.com
Source: knowinsiders.com
You can view more details on each measurement unit. You can view more details on each measurement unit. We assume you are converting between moles CH3OH and gram. 5844 g 5844 grmol 100 mol. Knowing how to convert grams to moles may be helpful in numerous chemical tasks eg finding the mole fraction of a solution.
 Source: study.com
Source: study.com
And 00 oC will be produced. Multiply the moles given by the substances molar mass. Convert grams to moles. One mole of. Mole of gold.
 Source: slidetodoc.com
Source: slidetodoc.com
You can view more details on each measurement unit. Molar mass of gold 19697 gmol. Mole of gold 005 mole. Sometimes a book will write out the word molal as in 0500-molal. 21008 3206 418 106076.
 Source: science.wonderhowto.com
Source: science.wonderhowto.com
You can view more details on each measurement unit. Therefore the molecular mass of H 2 SO 4 is. You can view more details on each measurement unit. Formula mass of acetic acid is 6005 amu moles grams formula mass moles 252 g acetic acid 6005 amu per mole of acetic acid moles 0420 moles Molarity M mol solute 1 liter of solution M 0420 moles acetic acid 0500 liter vinegar solution M 0839 molesL 0839 M. D One mole of water contains 12 mole of oxygen atoms.
 Source: youtube.com
Source: youtube.com
1 mole is equal to 1 moles Water or 1801528 grams. As you already know how the grams to moles conversion work find the number of moles. The mass of 005 mole of gold is 985 g. Where is the molar mass of the substance. D One mole of water contains 12 mole of oxygen atoms.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title what is 05 moles in grams by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.