Your What does the term g mol mean images are available in this site. What does the term g mol mean are a topic that is being searched for and liked by netizens now. You can Find and Download the What does the term g mol mean files here. Get all royalty-free photos and vectors.
If you’re looking for what does the term g mol mean images information related to the what does the term g mol mean keyword, you have pay a visit to the ideal site. Our site always provides you with suggestions for downloading the highest quality video and picture content, please kindly surf and locate more enlightening video content and graphics that fit your interests.
What Does The Term G Mol Mean. Updated on November 07 2019. The mol is defined from the massunit units which is defined as of the mass of a carbon-12 atom so that 1 u 1 gmol. 1 dozen 12 items similarly we use the mole to calculate the size of the smallest entities quantitatively. The basic unit of amount of substance adopted under the Systeme International dUnites.

Updated on November 07 2019. A gram mole is equal to the mass of one mole of substance Avogadros Number 6022 x 1023 of atoms ions molecules in grams. G mol means gram mole. The Wikipedia article on the mole states. 1 dozen 12 items similarly we use the mole to calculate the size of the smallest entities quantitatively. Gram molecule mole mol noun the molecular weight of a substance expressed in grams.
Molar concentration has the units molL or M.
1 dozen 12 items similarly we use the mole to calculate the size of the smallest entities quantitatively. It is the mass of a mole of a substance. It is denoted by ma. Updated on November 07 2019. Just like a dusin is 12 and a million is 1000000 1 mol is 6022 10 23. It has a unit of the unified mass unit u or the atomic mass unit.
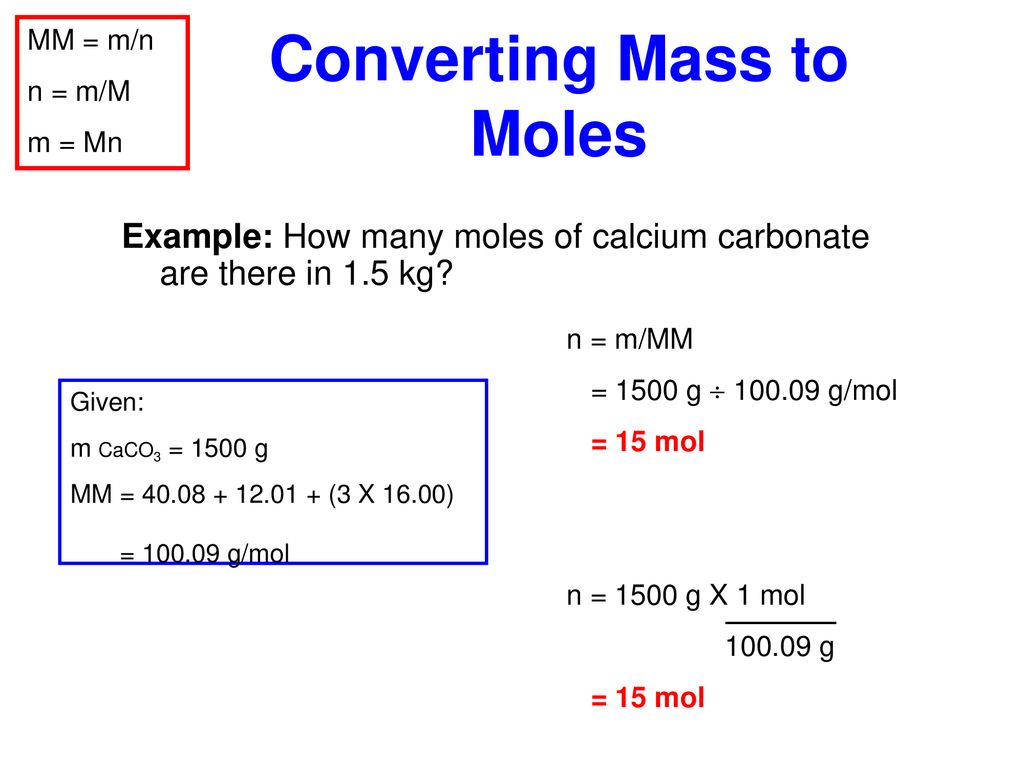 Source: slideplayer.com
Source: slideplayer.com
It has a unit of the unified mass unit u or the atomic mass unit. The term gram-atom abbreviated gat has been used for a related but distinct concept namely a quantity of a substance that contains Avogadros number of atoms whether isolated or combined in molecules. It has a unit of the unified mass unit u or the atomic mass unit. A gram mole is equal to the mass of one mole of substance Avogadros Number 6022 x 1023 of atoms ions molecules in grams. The mol is defined from the massunit units which is defined as of the mass of a carbon-12 atom so that 1 u 1 gmol.
 Source: khanacademy.org
Source: khanacademy.org
Learn about our Editorial Process. G mol means gram mole. The mol is defined from the massunit units which is defined as of the mass of a carbon-12 atom so that 1 u 1 gmol. The basic unit of amount of substance adopted under the Systeme International dUnites. Just as we take a standard value to calculate different things eg.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
G mol means gram mole. Gram molecule mole mol noun. Most chemists abbreviate mole and moles to mol and mols. Molar mass is the mass of a given substance divided by the amount of that substance measured in gmol. However the SI unit is kg mol1 which is very rare.
 Source: chemistrygod.com
Source: chemistrygod.com
Moles KCl 12 g KCl1 mol7455 g moles KCl 00161 mol. The symbol used for it is M. Thus for example 1 mole of ceMgB2 is 1 gram-molecule of ceMgB2 but 3 gram-atoms of ceMgB2. Gram molecule mole mol noun. The basic unit of amount of substance adopted under the Systeme International dUnites.
 Source: clutchprep.com
Source: clutchprep.com
A gram mole is equal to the mass of one mole of substance Avogadros Number 6022 x 1023 of atoms ions molecules in grams. Moles KCl 12 g KCl1 mol7455 g moles KCl 00161 mol. The symbol used for it is M. The term gram-atom abbreviated gat has been used for a related but distinct concept namely a quantity of a substance that contains Avogadros number of atoms whether isolated or combined in molecules. The standard unit for this is g mol1.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
It is the mass of a mole of a substance. Gram molecule mole mol noun. Molar refers to the unit of concentration molarity which is equal to the number of moles per liter of a solution. A gram mole is equal to the mass of one mole of substance Avogadros Number 6022 x 1023 of atoms ions molecules in grams. The atomic mass is the sum of the mass of protons neutrons and electrons.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
The standard unit for this is g mol1. G mol means gram mole. The term gram-atom abbreviated gat has been used for a related but distinct concept namely a quantity of a substance that contains Avogadros number of atoms whether isolated or combined in molecules. Although mass can be expressed as both amu and gmol gmol is the most useful system of units for laboratory chemistry. Mass of KCl mass of K mass of Cl mass of KCl 3910 g 3545 g mass of KCl 7455 gmol You have 12 grams of KCl so you need to find how many moles that is.

Gram molecule mole mol noun. It is denoted by ma. Just like a dusin is 12 and a million is 1000000 1 mol is 6022 10 23. Thus for example 1 mole of ceMgB2 is 1 gram-molecule of ceMgB2 but 3 gram-atoms of ceMgB2. The mol is defined from the massunit units which is defined as of the mass of a carbon-12 atom so that 1 u 1 gmol.
 Source: khanacademy.org
Source: khanacademy.org
Thus one water molecule weighs 1802 u whereas 1. A gram-mole of salt NaCl is 5844 grams. Molar mass is the mass of a given substance divided by the amount of that substance measured in gmol. The atomic mass is the sum of the mass of protons neutrons and electrons. The molecular weight of a substance expressed in grams.
 Source: sciencetrends.com
Source: sciencetrends.com
The atomic mass is the sum of the mass of protons neutrons and electrons. Thus for example 1 mole of ceMgB2 is 1 gram-molecule of ceMgB2 but 3 gram-atoms of ceMgB2. The mol is defined from the massunit units which is defined as of the mass of a carbon-12 atom so that 1 u 1 gmol. Although mass can be expressed as both amu and gmol gmol is the most useful system of units for laboratory chemistry. For example the atomic mass of titanium is 4788 amu or 4788 gmol.
 Source: study.com
Source: study.com
Molar mass is the mass of a given substance divided by the amount of that substance measured in gmol. Although mass can be expressed as both amu and gmol gmol is the most useful system of units for laboratory chemistry. A gram-mole of salt NaCl is 5844 grams. The term gram-atom abbreviated gat has been used for a related but distinct concept namely a quantity of a substance that contains Avogadros number of atoms whether isolated or combined in molecules. The atomic mass is the sum of the mass of protons neutrons and electrons.
 Source: wikihow.com
Source: wikihow.com
Weight-average molecular weight M w gmol virial coefficient A 2 mol cm 3 g 2 radius of gyration R g z A polymer structure anisotropy polydispersity Measurement of scattered light intensities from dilute polymer solutions dependent. A gram-mole of salt NaCl is 5844 grams. Weight-average molecular weight M w gmol virial coefficient A 2 mol cm 3 g 2 radius of gyration R g z A polymer structure anisotropy polydispersity Measurement of scattered light intensities from dilute polymer solutions dependent. Although mass can be expressed as both amu and gmol gmol is the most useful system of units for laboratory chemistry. The term gram-atom abbreviated gat has been used for a related but distinct concept namely a quantity of a substance that contains Avogadros number of atoms whether isolated or combined in molecules.
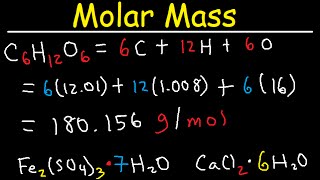 Source: youtube.com
Source: youtube.com
Most chemists abbreviate mole and moles to mol and mols. A gram-mole of salt NaCl is 5844 grams. The Wikipedia article on the mole states. For example the atomic mass of titanium is 4788 amu or 4788 gmol. Learn about our Editorial Process.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
It is the mass of a mole of a substance. The symbol used for it is M. Weight-average molecular weight M w gmol virial coefficient A 2 mol cm 3 g 2 radius of gyration R g z A polymer structure anisotropy polydispersity Measurement of scattered light intensities from dilute polymer solutions dependent. Molar mass is the mass of a given substance divided by the amount of that substance measured in gmol. The basic unit of amount of substance adopted under the Systeme International dUnites.
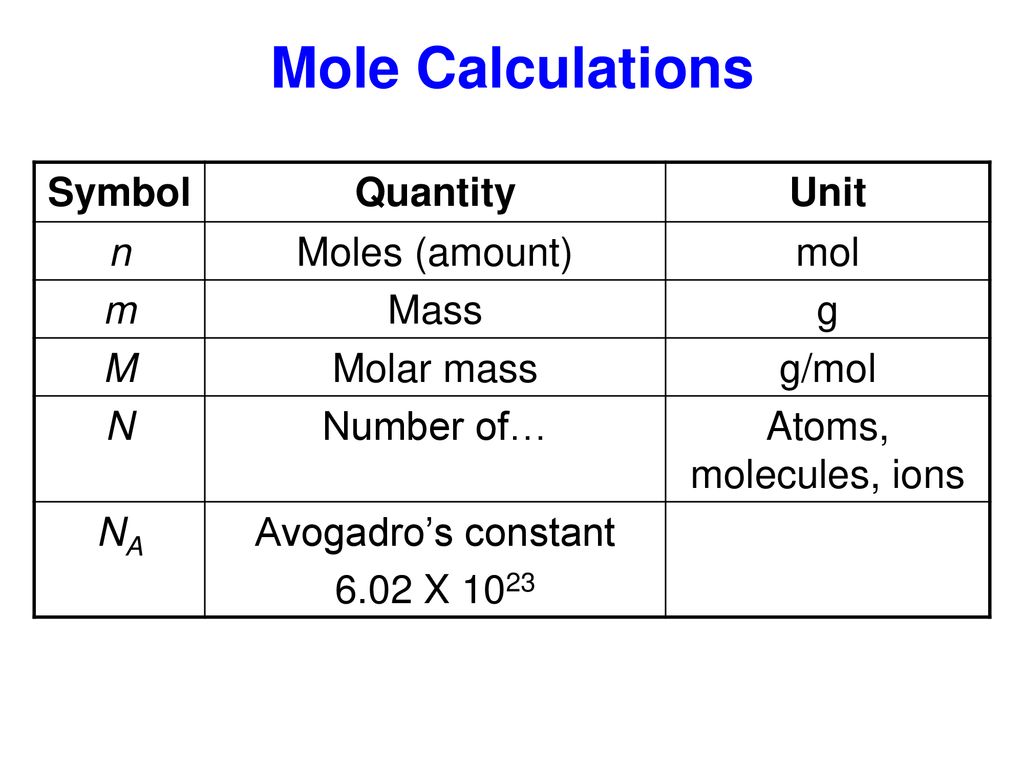 Source: slideplayer.com
Source: slideplayer.com
Updated on November 07 2019. G mol means gram mole. It is the mass of a mole of a substance. Thus for example 1 mole of ceMgB2 is 1 gram-molecule of ceMgB2 but 3 gram-atoms of ceMgB2. Just like a dusin is 12 and a million is 1000000 1 mol is 6022 10 23.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title what does the term g mol mean by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






