Your What does one mole of water molecule represent images are available. What does one mole of water molecule represent are a topic that is being searched for and liked by netizens today. You can Get the What does one mole of water molecule represent files here. Get all free photos and vectors.
If you’re looking for what does one mole of water molecule represent pictures information linked to the what does one mole of water molecule represent interest, you have pay a visit to the right blog. Our website always gives you suggestions for seeing the highest quality video and image content, please kindly surf and find more enlightening video articles and graphics that match your interests.
What Does One Mole Of Water Molecule Represent. If I have 6022xx1023 one mole of 12C atoms I have a mass of 12g precisely. Define atomic mass unit. This conversation is already closed by Expert. Divide the mass of the compound in grams by the molar mass you just calculated.
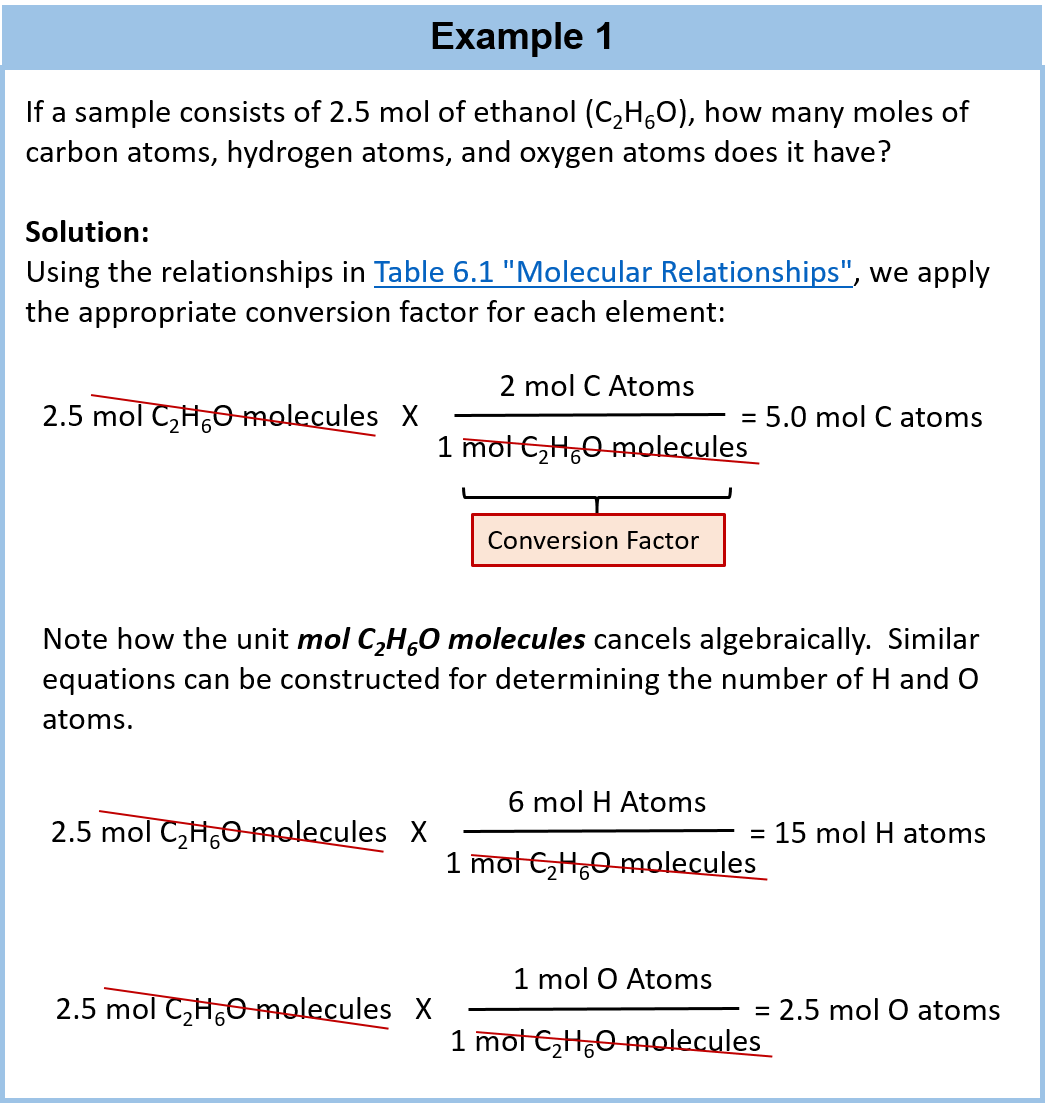 Chapter 6 Quantities In Chemical Reactions Chemistry From wou.edu
Chapter 6 Quantities In Chemical Reactions Chemistry From wou.edu
We have calculated the mass of one molecule of water as 29891023g. And said 2 moles of water decomposed to form 2 moles of molecular hydrogen and 1 mole of molecular oxygen. So 1 mole H2O 120441024 hydrogen atoms. Oxygen a becomes slightly positively charged due to the protons on the water molecule. B One mole of water contains the same number of molecules as one mole of ammonia. Divide the mass of the compound in grams by the molar mass you just calculated.
C The mole concept makes a connection between the mass of a substance and the number of.
18 amu is the mass of one molecule of water. A mole is a convenient counting unit whenever one is dealing with numbers of atoms or molecules. This is not the molecular mass but the molar mass ie. Double that for 2 moles and there are. To use our old friend water as an example. Therefore 2 mole H2O will have 240881024 hydrogen atoms.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
The mass of one mole of H2O molecules. Atomic mass is the number of grams per mole of the element. This means 1 mole of hydrogen weighs 10079 grams and 1 mole of oxygen weighs. Since one mole is defined as 6022 x 1023 and there are two hydrogen per molecule that would mean that there are exactly 12044 x 1023 molecules of hydrogen in one mole of water. Which Mole has the largest mass.
 Source: chem.fsu.edu
Source: chem.fsu.edu
1 mole of Na atoms 23 g of Na atoms 6023 X 1023 atoms of Na. Which molecule has the greatest mass and atomic size. Atomic mass unit of an element is one twelfth 112th of the mass of one atom of carbon-12. The valency of sulphur in H 2 S SO 2 and SO 3 are 2 4 and 6 respectively. Its 6022 x 1023 molecules or atoms or ions whatever we are talking about.
 Source: slideplayer.com
Source: slideplayer.com
Its molecular weight in gram eg. I understand that the one mole of atoms is equal to 602 10 23 atoms. Convert grams Water to moles or moles Water to grams. Double that for 2 moles and there are. Which Mole has the largest mass.
 Source: toppr.com
Source: toppr.com
Atoms and Molecules Class 9 Extra Questions Very Short Answer Type. And said 2 moles of water decomposed to form 2 moles of molecular hydrogen and 1 mole of molecular oxygen. This is the molar mass of the compound. The mass of oxygen equal to one mole of oxygen is 15998 grams and the mass of one mole of hydrogen is 1008 g. If we have one.
 Source: intro.chem.okstate.edu
Source: intro.chem.okstate.edu
The reactants shown schematically below represent iron oxide Fe2O3 and carbon monoxide CO. Correspondingly what does 1 mole of water weigh. How many moles of water does 602 x10 23 molecules represent. Define atomic mass unit. This is not the molecular mass but the molar mass ie.
 Source: slideplayer.com
Source: slideplayer.com
Double that for 2 moles and there are 24088 x 1023 hydrogen atoms in one mole of water. If I have 6022xx1023 one mole of 12C atoms I have a mass of 12g precisely. However when I used various resources to learn about stoichiometry they showed me a balanced equation. 14 of a mole x 602 x 1023 atomsmole approximately 15 x 1023 atoms. Write the valency of sulphur in H 2 S SO 2 and SO 3.
 Source: socratic.org
Source: socratic.org
Hence one mole molecule of a compound would always contain 6022 10 23 molecules. Since one mole is defined as 6022 x 1023 and there are two hydrogen per molecule that would mean that there are exactly 12044 x 1023 molecules of hydrogen in one mole of water. In chemistry one mole of an element or substance is equal to. H2O 12 16 18 amu. The mole is thus the link between the sub-micro world of atoms and molecules which we cannot see but whose existence we can infer with the macro world of grams and kilograms and litres that which can measure out in a lab by various means ie.
 Source: youtube.com
Source: youtube.com
From the periodic table we see the atomic weight of hydrogen is 10079 and the atomic weight of oxygen is 159994. However when I used various resources to learn about stoichiometry they showed me a balanced equation. One mole of any atom consists of the same number of particles. The valency of sulphur in H 2 S SO 2 and SO 3 are 2 4 and 6 respectively. Its molecular weight in gram eg.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
We have calculated the mass of one molecule of water as 29891023g. One mole of Water is composed of 1 mole of Oxygen and two moles of Hydrogen. As a molecule of water consists of two atoms of hydrogen and one of oxygen. 1 mole of Na atoms 23 g of Na atoms 6023 X 1023 atoms of Na. Which molecule has the greatest mass and atomic size.
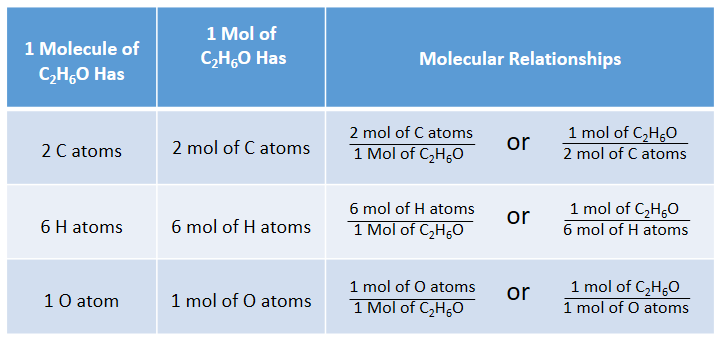 Source: wou.edu
Source: wou.edu
However when I used various resources to learn about stoichiometry they showed me a balanced equation. A calorie was originally defined as the amount of energy required to raise the temperature of one gram of water by one degree Celsius. Similarly 1 mole of water H 2 O molecules 18 g of water molecule 6023 X 1023 molecules of water. A group of atoms so united and combined by chemical affinity that they form a complete integrated whole being the smallest portion of any particular compound that can exist in a free state. 18 gm per mol is the mass of one mole of water.
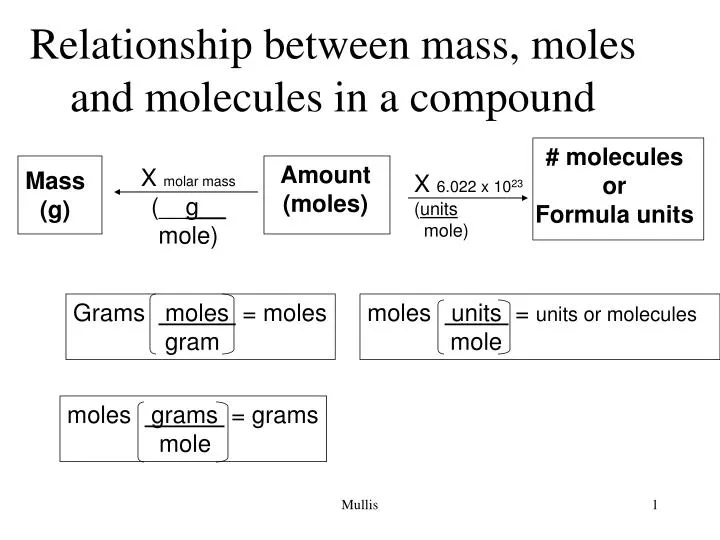 Source: slideserve.com
Source: slideserve.com
Since one mole is defined as 6022 x 1023 and there are two hydrogen per molecule that would mean that there are exactly 12044 x 1023 molecules of hydrogen in one mole of water. Hence one mole of. Which molecule has the greatest mass and atomic size. D One mole of any compound contains one mole of atoms. This is not the molecular mass but the molar mass ie.

So 1 mole H2O 120441024 hydrogen atoms. So 1 mole H2O 120441024 hydrogen atoms. Atomic mass is the number of grams per mole of the element. If I have 6022xx1023 one mole of 12C atoms I have a mass of 12g precisely. The mass of oxygen equal to one mole of oxygen is 15998 grams and the mass of one mole of hydrogen is 1008 g.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
This is the molar mass of the compound. Since one mole is defined as 6022 x 1023 and there are two hydrogen per molecule that would mean that there are exactly 12044 x 1023 molecules of hydrogen in one mole of water. Its molecular weight in gram eg. This is not the molecular mass but the molar mass ie. 18 gram water 1 mole water.
 Source: slideplayer.com
Source: slideplayer.com
From the periodic table we see the atomic weight of hydrogen is 10079 and the atomic weight of oxygen is 159994. 1 mole 60221023 atoms. Molar mass of H2O 1801528 gmol. C The mole concept makes a connection between the mass of a substance and the number of. Define atomic mass unit.
 Source: wou.edu
Source: wou.edu
Molar mass of H2O 1801528 gmol. 2 H X 2 O 2 H X 2 O X 2. How many moles of water does 602 x10 23 molecules represent. This is not the molecular mass but the molar mass ie. Convert grams Water to moles or moles Water to grams.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title what does one mole of water molecule represent by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






