Your What does moles per liter mean images are ready. What does moles per liter mean are a topic that is being searched for and liked by netizens now. You can Get the What does moles per liter mean files here. Find and Download all free photos and vectors.
If you’re looking for what does moles per liter mean images information linked to the what does moles per liter mean interest, you have pay a visit to the right blog. Our site always provides you with suggestions for seeking the highest quality video and picture content, please kindly hunt and find more enlightening video content and graphics that fit your interests.
What Does Moles Per Liter Mean. The kilogram-mole is the most uninteresting thing in chemistry. 25M means 25 moles of sulfuric acid per one liter of solution. Units of Molarity. Molarity M indicates the number of moles of solute per liter of solution molesLiter and is one of the most common units used to measure the concentration of a solution.
 Moles What Is Molar Mass Molar Mass Is From slidetodoc.com
Moles What Is Molar Mass Molar Mass Is From slidetodoc.com
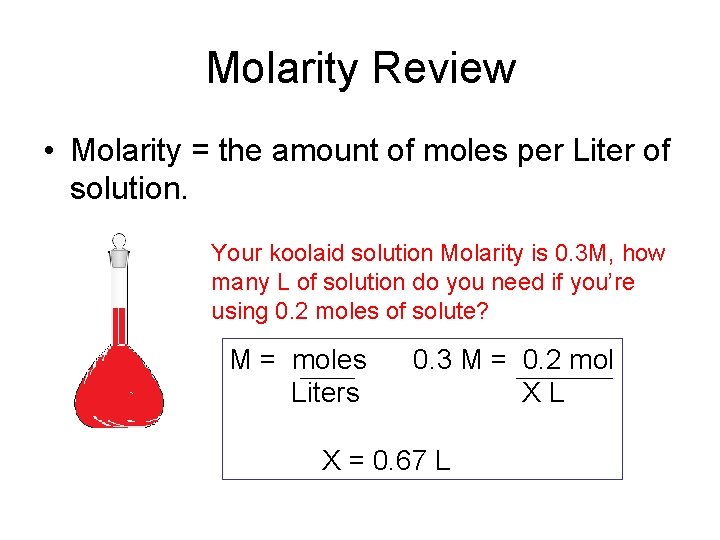
Moles mol Molarity M x Volume L 05 x 2. The mole may also be used to measure the amount of atoms ions electrons or other entities. MgL or mgL-1 is how many milligrams per there are in a 1 Liter solution. The classic mole is also. Thus 1000 g x 1 mol18 g 556 mol. X 010012 833.
Thus 1000 g x 1 mol18 g 556 mol.
A solution using these units is called a molal solution eg 01 m NaOH is a 01 molal solution of sodium hydroxide. The classic mole is also. M o l L 1 is more conveniently written m o l L. In chemistry the most commonly used unit for molarity is the number of moles per liter having the unit symbol molL or moldm3 in SI unit. Its such a common unit it has its own symbol which is a capital letter M. Units of Molarity.
 Source: redbubble.com
Source: redbubble.com
One mole contains exactly 6022 140 76 10 23 elementary entities. 25M means 25 moles of sulfuric acid per one liter of solution. Mol or ML is how many mols weight of a substance there is in 1 Liter. The units are mole per liter. But remember from above.
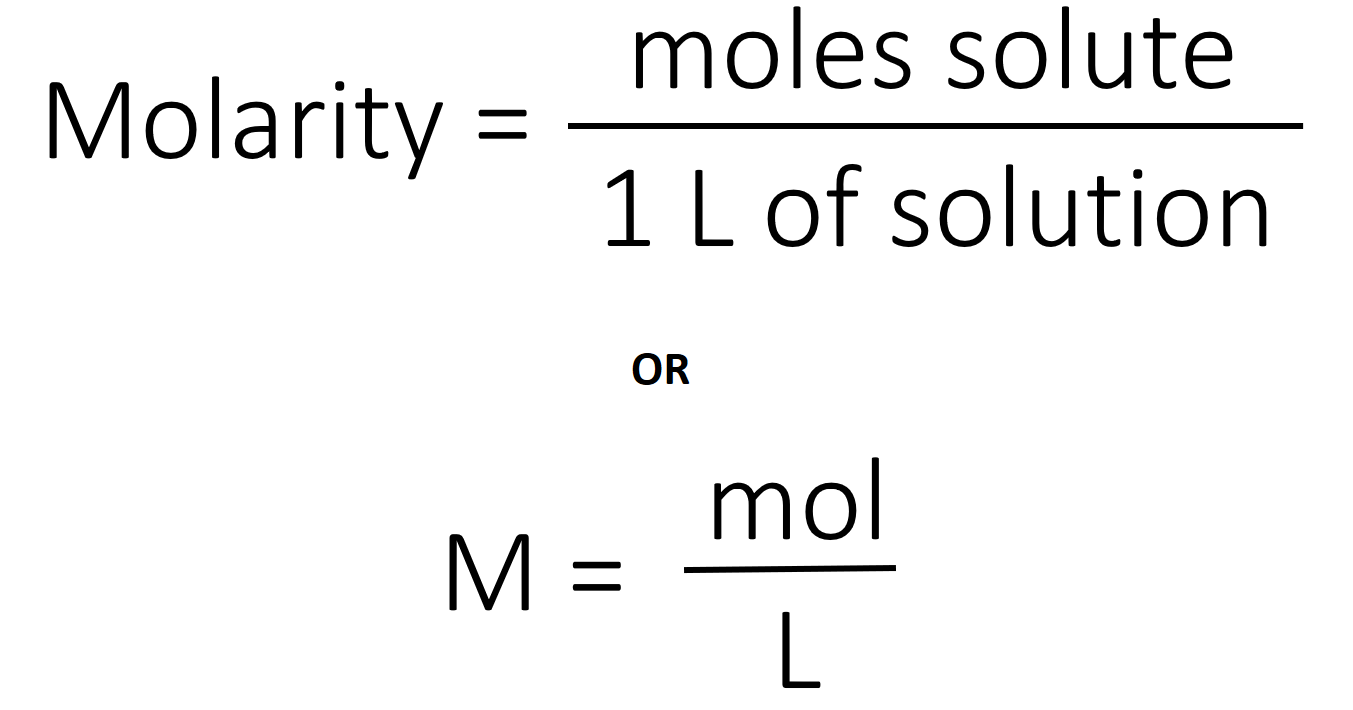 Source: wou.edu
Source: wou.edu
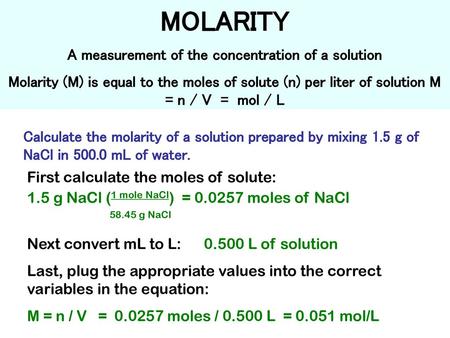
Molarity is defined as the number of moles of solute in exactly 1 liter 1 L of the solution. In chemistry molar concentration or molarity is defined as moles of solute per total liters of solution. The concentration of a solution is commonly expressed by its molarity defined as the amount of dissolved substance in moles per unit volume of solution for which the unit typically used is moles per litre molL commonly abbreviated M. Show activity on this post. Uppercase M is molarity which is moles of solute per liter of solution not solvent.
 Source: slideplayer.com
Source: slideplayer.com
X 010012 833. The classic mole is also. Of millimoles per liter or micromoles per liter Refers to the chemical activity of an electrolyte Is related to the total number of ionic charges in solution and considers the. Molarity is expressed in units of moles per liter molL. Molarity is defined as the number of moles of solute in exactly 1 liter 1 L of the solution.
 Source: reddit.com
Source: reddit.com
To get 01 moles of HCl requires 833 mL con HCl. Just use the mole. The molar mass of sulfuric acid is 9809 gmol. This is an important distinction. A solution that has the concentration 5 molL would be called a 5 M solution or said to have a.
 Source: redbubble.com
Source: redbubble.com
This is for 1 L of solution so you need to dilute that 833 mL of con HCL up to a total volume of 1 liter meaning you add the 833 mL con HCl to 99167 mL water. This is for 1 L of solution so you need to dilute that 833 mL of con HCL up to a total volume of 1 liter meaning you add the 833 mL con HCl to 99167 mL water. Molal designating a solution containing one mole of solute per kilogram of solvent molar containing one mole of a substance molar designating a solution containing one mole of solute per liter of solution Sense 2. Calculate the grams of the solvent. This is an important distinction.
 Source: pinterest.com
Source: pinterest.com
So for 1 liter of solution you need 01 moles of HCl. They are equivalent since 1 dm 3 1 liter. The definition of a mole is as follows. A molar solution is defined as an aqueous solution that contains 1 mole gram-molecular weight of a compound dissolved in 1 liter of a solution. To multiply a kilowatt with an hour makes a physically meaning unit energy and hence the term kilowatt-hour is okay.
 Source: redbubble.com
Source: redbubble.com
Calculate the grams of the solvent. 1540 grams of solution - 245225 grams of solute 1294775grams or 1294775 kg solvent. Of millimoles per liter or micromoles per liter Refers to the chemical activity of an electrolyte Is related to the total number of ionic charges in solution and considers the. Show activity on this post. Answer 1 of 2.
 Source: slidetodoc.com
Source: slidetodoc.com
A solution using these units is called a molal solution eg 01 m NaOH is a 01 molal solution of sodium hydroxide. Uppercase M is molarity which is moles of solute per liter of solution not solvent. Convert 25 moles to grams. Thus 1000 g x 1 mol18 g 556 mol. Moles mol Molarity M x Volume L 05 x 2.
 Source: slideplayer.com
Source: slideplayer.com
And since the molar mass of water is 18 g per mol 18 gmol we can convert the 1000 g of water to moles of water by multiplying 1000 g of water by the ratio 1 mol18 g. The referenced site is using the regular notation. The molar mass of sulfuric acid is 9809 gmol. A millimole is one-thousandth of a mole. Show activity on this post.
 Source: allacronyms.com
Source: allacronyms.com
Thus 1000 g x 1 mol18 g 556 mol. Mol or ML is how many mols weight of a substance there is in 1 Liter. Molarity M indicates the number of moles of solute per liter of solution molesLiter and is one of the most common units used to measure the concentration of a solution. X 010012 833. Since the unified atomic mass unit symbol.
 Source: redbubble.com
Source: redbubble.com
Show activity on this post. Mol or ML is how many mols weight of a substance there is in 1 Liter. The classic mole is also. Molal designating a solution containing one mole of solute per kilogram of solvent molar containing one mole of a substance molar designating a solution containing one mole of solute per liter of solution Sense 2. They are equivalent since 1 dm 3 1 liter.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Moles are simply a unit of mass and are abbreviated as mol. 1540 grams of solution - 245225 grams of solute 1294775grams or 1294775 kg solvent. A solution with a concentration of 1 molL is said to be 1 molar commonly designated as 1 M. The units are mole per liter. The classic mole is also.
 Source: fashionloveshirts.com
Source: fashionloveshirts.com
The definition of a mole is as follows. Calculate the grams of the solvent. The classic mole is also. And since the molar mass of water is 18 g per mol 18 gmol we can convert the 1000 g of water to moles of water by multiplying 1000 g of water by the ratio 1 mol18 g. A solution using these units is called a molal solution eg 01 m NaOH is a 01 molal solution of sodium hydroxide.
 Source: amazon.com
Source: amazon.com
This set of units is referred to as molarity and is a common measure of concentration for a solute dissolved in a solvent. The concentration of a solution is commonly expressed by its molarity defined as the amount of dissolved substance in moles per unit volume of solution for which the unit typically used is moles per litre molL commonly abbreviated M. MgL or mgL-1 is how many milligrams per there are in a 1 Liter solution. Millimoles Per Litre mmolL A mole is an amount of a substance that contains a large number 6 followed by 23 zeros of molecules or atoms. If one mole of solute is dissolved in 1 liter of solution then the molarity of the solution is said to be 1M.
 Source: allacronyms.com
Source: allacronyms.com
A solution that has the concentration 5 molL would be called a 5 M solution or said to have a. Molal designating a solution containing one mole of solute per kilogram of solvent molar containing one mole of a substance molar designating a solution containing one mole of solute per liter of solution Sense 2. Moles are simply a unit of mass and are abbreviated as mol. In other words the solution has a concentration of 1 molL or a molarity of 1 1M. But remember from above.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title what does moles per liter mean by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






