Your What does grams per mole mean images are available in this site. What does grams per mole mean are a topic that is being searched for and liked by netizens now. You can Download the What does grams per mole mean files here. Find and Download all free images.
If you’re searching for what does grams per mole mean images information linked to the what does grams per mole mean topic, you have pay a visit to the ideal site. Our website always gives you suggestions for refferencing the highest quality video and image content, please kindly hunt and locate more enlightening video content and images that fit your interests.
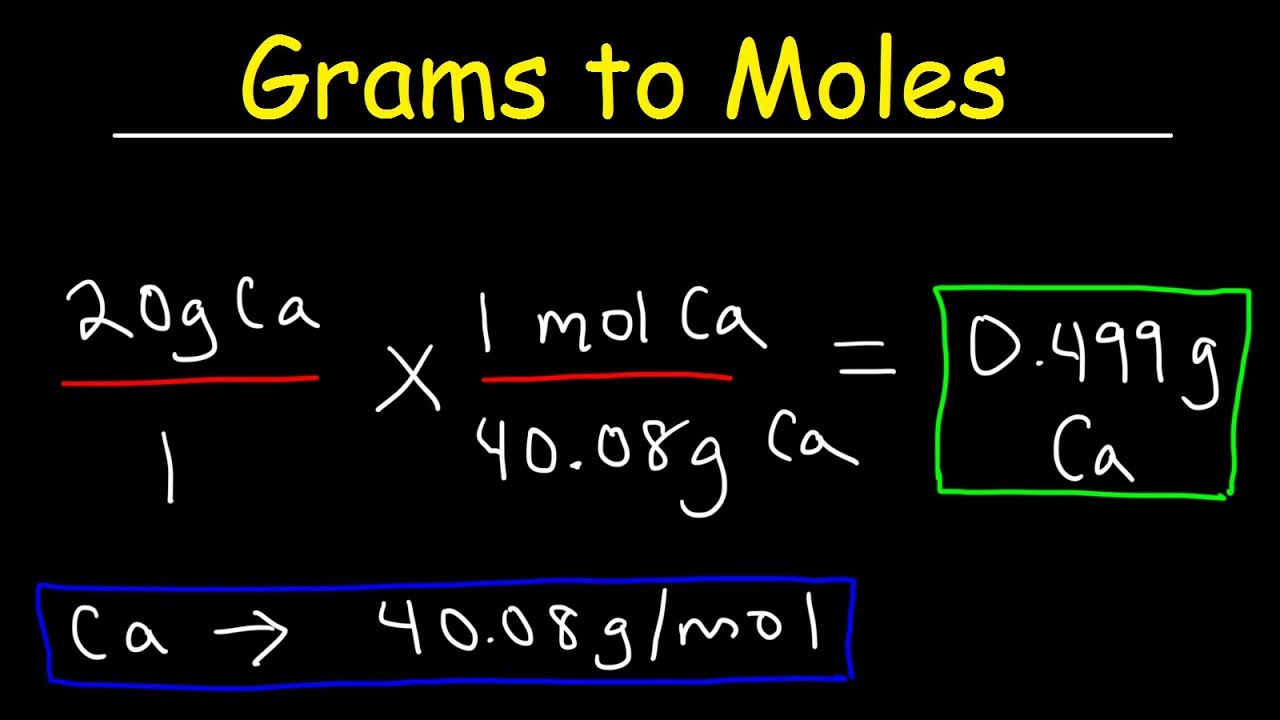
What Does Grams Per Mole Mean. For example iron Fe has a. So your first example molL-1 s-1 is not correct - it would actually be written as mol L-1 s-1 OR molL s. The unit used to measure is grams per mole. So one gram mole of CO2 is 44 grams.
 Mole Easy Science Mole Day Easy Science Chemistry From pinterest.com
Mole Easy Science Mole Day Easy Science Chemistry From pinterest.com
Mole percent is the percentage that the moles of a particular component are of the total moles that are in a mixture. That said to find the mass of one ATOM we need to convert from moles to atoms as follows. So one gram mole of O2 is 32 grams. It can be calculated using the elements atomic weight from the periodic table and expressed in grams. 1008 gramsmole Hydrogen 1 mole6022x10 23 atoms 167 x 10-24 grams. This is the weight in grams of 1 mole of the compound.
Molecular mass of substance.
The mass of one mole of carbon-12 atoms is exactly 12 grams. The definition of atomic mass the mole and molar mass are all directly or indirectly related to carbon-12. The molar mass also known as molecular weight is the sum of the total mass in grams of all the atoms that make up a mole of a particular molecule. A gram per mole g mol-1 is a molar mass or the mass of a mole of things. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. This property simplifies many chemical computations.
 Source: pinterest.com
Source: pinterest.com
Molecular mass of substance. The mass of a unit mole of an element appears to equal the gram atomic mass. But even though the weight is different the two moles contain the exact same number of molecules 602 x 10 to the 23rd power. Mole percent is the percentage that the moles of a particular component are of the total moles that are in a mixture. For NaCl the molar mass is 5844 gmol.
 Source: pinterest.com
Source: pinterest.com
So your first example molL-1 s-1 is not correct - it would actually be written as mol L-1 s-1 OR molL s. For example the mean molecular weight of water is 18015 atomic mass units amu so one mole of water weight 18015 grams. What is grams per mole equal to. 1 millimole Fe2O3 15969 1000 01597 grams 15969 milligrams. So one gram mole of O2 is 32 grams.

Moles mol Molarity M x Volume L 05 x 2. A gram-mole of salt NaCl is 5844 grams. The term gram-atom abbreviated gat has been used for a related but distinct concept namely a quantity of a substance that contains Avogadros number of atoms whether isolated or combined in molecules. Since the unified atomic mass unit symbol. 2 55845 3 16000 11169 4800 15969.
 Source: pinterest.com
Source: pinterest.com
For the molecule CO2 the molecular weight is 121616 or 44. Mole fraction chi the Greek letter chi is the number of moles of a given component of a mixture divided by the total number of moles in the mixture. For oxygen which exists in air as the molecule O2 the molecular weight is 16x2 or 32. So your first example molL-1 s-1 is not correct - it would actually be written as mol L-1 s-1 OR molL s. It is also sometimes written as molLs but the double division is ambiguous and should be avoided unless parentheses are used.
 Source: pinterest.com
Source: pinterest.com
These numbers are either in atomic mass units amu or in grams per mole of atoms. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. That said to find the mass of one ATOM we need to convert from moles to atoms as follows. 1 Gram-mole g-mol 1 000 Millimole mmol - Measurement calculator that can be. So one gram mole of CO2 is 44 grams.
 Source: in.pinterest.com
Source: in.pinterest.com
Mole percent is the percentage that the moles of a particular component are of the total moles that are in a mixture. U or Da is defined as 112 of the mass of the 12 C atom it follows that the molar mass of a substance measured in grams per mole is numerically equal to its mean atomic or molecular mass measured in Da. So your first example molL-1 s-1 is not correct - it would actually be written as mol L-1 s-1 OR molL s. This is the weight in grams of 1 mole of the compound. For NaCl the molar mass is 5844 gmol.
 Source: youtube.com
Source: youtube.com
Add the weight of each atom in the compound. But even though the weight is different the two moles contain the exact same number of molecules 602 x 10 to the 23rd power. So a mole of a molecule like hydrogen H with an atomic weight of 1 is one gram. One gram mole of anything including gases is the molecular weight of a molecule in grams. For example iron Fe has a.
 Source: pinterest.com
Source: pinterest.com
This means that 1 MOLE of hydrogen atoms will weigh 1008 grams. A gram per mole g mol-1 is a molar mass or the mass of a mole of things. Moles mol Molarity M x Volume L 05 x 2. Often called gram-molecular weight A mass of a substance in grams numerically equal to its molecular weight. Mole percent is the percentage that the moles of a particular component are of the total moles that are in a mixture.
 Source: br.pinterest.com
Source: br.pinterest.com
For the molecule CO2 the molecular weight is 121616 or 44. Number of atoms present in one mole. This is the weight in grams of 1 mole of the compound. We assume you are converting between moles In and gram. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance.
 Source: pinterest.com
Source: pinterest.com
This is the weight in grams of 1 mole of the compound. 1 millimole Fe2O3 15969 1000 01597 grams 15969 milligrams. So a mole of a molecule like hydrogen H with an atomic weight of 1 is one gram. It can be calculated using the elements atomic weight from the periodic table and expressed in grams. A gram-mole of salt NaCl is 5844 grams.
 Source: in.pinterest.com
Source: in.pinterest.com
Add the weight of each atom in the compound. A mole is the atomic weight of a molecule of the chemical in grams. The mass of one mole of carbon-12 atoms is exactly 12 grams. Mole is the unit of measurement for the amount of substance. This property simplifies many chemical computations.
 Source: pinterest.com
Source: pinterest.com
The number 6022140761023 the Avogadro number was chosen so that the mass of one mole of a chemical compound in grams is numerically equal for most practical purposes to the average mass of one molecule of the compound in daltons. A mole is the atomic weight of a molecule of the chemical in grams. Thus for example 1 mole of M g B X 2 is 1 gram-molecule of M g B X 2 but 3 gram-atoms of M g B X 2. Mass g No. Therefore the mole is a unit for that physical quantity.
 Source: pinterest.com
Source: pinterest.com
So one gram mole of CO2 is 44 grams. For oxygen which exists in air as the molecule O2 the molecular weight is 16x2 or 32. The molar mass also known as molecular weight is the sum of the total mass in grams of all the atoms that make up a mole of a particular molecule. The number 6022140761023 the Avogadro number was chosen so that the mass of one mole of a chemical compound in grams is numerically equal for most practical purposes to the average mass of one molecule of the compound in daltons. 1 Gram-mole g-mol 1 000 Millimole mmol - Measurement calculator that can be.
 Source: pinterest.com
Source: pinterest.com
It is also sometimes written as molLs but the double division is ambiguous and should be avoided unless parentheses are used. U or Da is defined as 112 of the mass of the 12 C atom it follows that the molar mass of a substance measured in grams per mole is numerically equal to its mean atomic or molecular mass measured in Da. The definition of atomic mass the mole and molar mass are all directly or indirectly related to carbon-12. One gram mole of anything including gases is the molecular weight of a molecule in grams. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.

For example the mean molecular weight of water is 18015 atomic mass units amu so one mole of water weight 18015 grams. For example the mean molecular weight of water is 18015 atomic mass units amu so one mole of water weight 18015 grams. So one gram mole of O2 is 32 grams. For oxygen which exists in air as the molecule O2 the molecular weight is 16x2 or 32. Its molar mass is exactly 12 grams per mole.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title what does grams per mole mean by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.