Your What does grams per mol mean images are ready in this website. What does grams per mol mean are a topic that is being searched for and liked by netizens today. You can Download the What does grams per mol mean files here. Get all free vectors.
If you’re searching for what does grams per mol mean pictures information connected with to the what does grams per mol mean keyword, you have pay a visit to the right site. Our site always gives you suggestions for seeking the maximum quality video and image content, please kindly hunt and find more enlightening video articles and graphics that fit your interests.
What Does Grams Per Mol Mean. N_t n_a. This quantity is sometimes referred to as the chemical amount. Thus for example the average mass of a molecule of water is about 180153 daltons and the molar mass of water is about 180153 gmol. The only difference is that gram molecular mass specifies the mass unit to be used.
 How To Convert Grams To Moles 8 Steps With Pictures Wikihow From wikihow.com
How To Convert Grams To Moles 8 Steps With Pictures Wikihow From wikihow.com
Gram molecular mass is the same as molar mass. MOLE FRACTION Lets start with the definition of mole fraction. The main distinction is that the gram molecular mass implies that the mass unit must be used. The molar mass also known as molecular weight is the sum of the total mass in grams of all the atoms that make up a mole of a particular molecule. Mole fraction chi the Greek letter chi is the number of moles of a given component of a mixture divided by the total number of moles in the mixture. Its the same as molar mass.
Gram molecular mass is the mass in grams of one mole of a molecular substance.
The official SI symbols are gcm 3 gcm 3 or g cm 3. This is the weight in grams of 1 mole of the compound. Use this page to learn how to convert between moles NO and gram. Calculate the grams of the solvent. A substance is something that has mass and occupies space. Often called gram-molecular weight A mass of a substance in grams numerically equal to its molecular weight.

Convert 25 moles to grams. Add the weight of each atom in the compound. This gives 1540 grams of solution. The answer is 247. MOLE FRACTION Lets start with the definition of mole fraction.
 Source: wikihow.com
Source: wikihow.com
Convert 25 moles to grams. What is grams per mole equal to. Gram molecular mass is the same as molar mass. 2 55845 3 16000 11169 4800 15969. If it were mol L-1 s-2 this would mean moles per litre per second per second.
 Source: br.pinterest.com
Source: br.pinterest.com
What does kJ mol mean kjmol is the abbreviation of kilojoules per mole which is a term used to describe the amount of energy in kilojoules that is put into a number of moles of atoms. This gives 1540 grams of solution. U or Da is defined as 112 of the mass of the 12 C atom it follows that the molar mass of a substance measured in grams per mole is numerically equal to its mean atomic or molecular mass measured in Da. A substance is something that has mass and occupies space. Any percentage form means the amount of the specific component to the total using the same units.
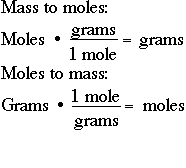 Source: socratic.org
Source: socratic.org
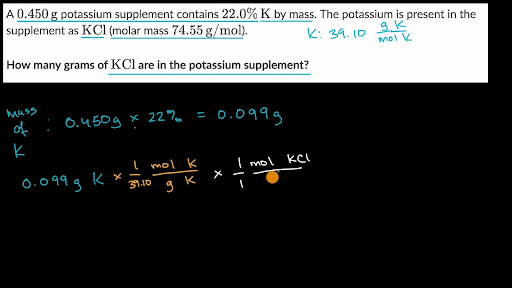
How many grams Cm in 1 mol. Use this page to learn how to convert between moles NO and gram. Often called gram-molecular weight A mass of a substance in grams numerically equal to its molecular weight. Gram molecular mass may be reported in grams or grams per mole gmol. A substance is something that has mass and occupies space.
 Source: wikihow.com
Source: wikihow.com
If it were mol L-1 s-2 this would mean moles per litre per second per second. 1 millimole Fe2O3 15969 1000 01597 grams 15969 milligrams. In some cases particularly in situations involving acid-base chemistry the solution concentration is expressed in normality N or C NNormality is defined as the number of equivalent weights or simply equivalents eq of solute dissolved per liter of solution equivalentsL N Equation 1Normality is used in place of molarity because often 1 mole of. A 1M solution would consist of 3423 grams sucrose in one liter final volume. To make 200 milliliters of your solution multiply gramsliter by liters needed.
 Source: pinterest.com
Source: pinterest.com
10 mol means 10 moles of that components mixed with 90 mole of the rest of components. What is grams per mole equal to. The mole was defined in such a way that the molar mass of a compound in gmol is numerically equal for all practical purposes to the average mass of one molecule in daltons. A 1M solution would consist of 3423 grams sucrose in one liter final volume. Gram molecular mass is the mass in grams of one mole of a molecular substance.
What does kJ mol mean kjmol is the abbreviation of kilojoules per mole which is a term used to describe the amount of energy in kilojoules that is put into a number of moles of atoms. A it is one-millionth of a gram per gram of sample solution. This property simplifies many chemical computations. Therefore the mole is a unit for that physical quantity. Often called gram-molecular weight A mass of a substance in grams numerically equal to its molecular weight.
 Source: youtube.com
Source: youtube.com
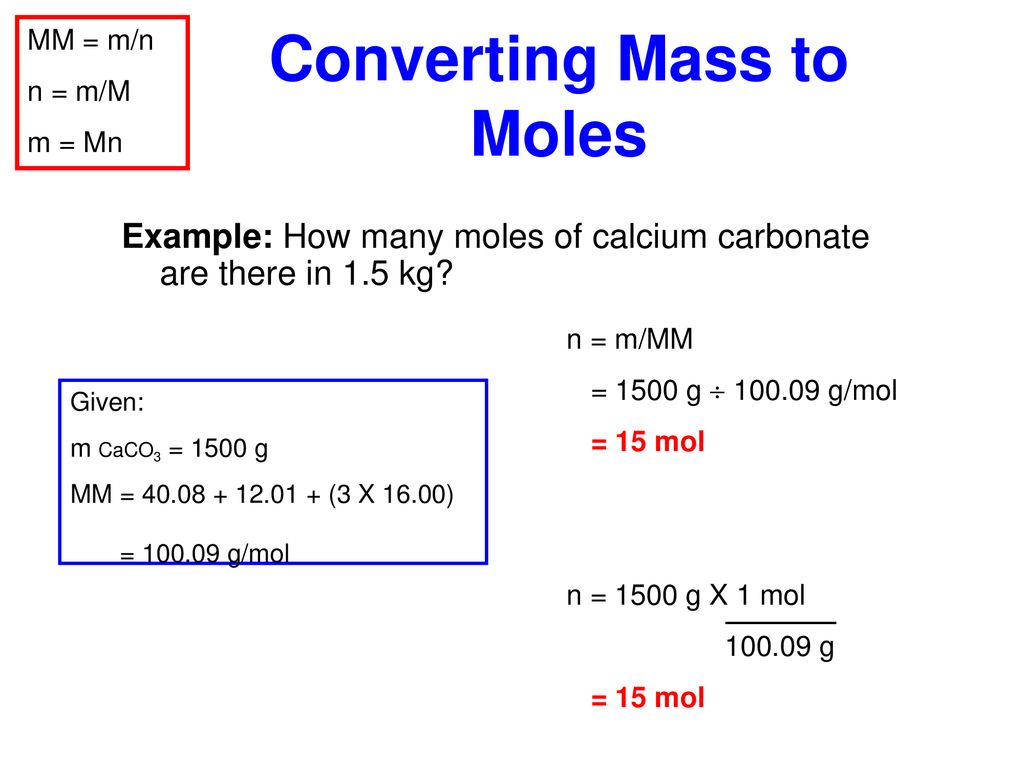
The gram per cubic centimetre is a unit of density in the CGS system commonly used in chemistry defined as mass in grams divided by volume in cubic centimetres. The official SI symbols are gcm 3 gcm 3 or g cm 3. Gram molecular mass is the mass in grams of one mole of a molecular substance. This quantity is sometimes referred to as the chemical amount. Note that rounding errors may occur so always check the results.
 Source: slideplayer.com
Source: slideplayer.com
Take 007 molesliter times 3423 grams per mole and you have 2396 grams needed per liter. Take 007 molesliter times 3423 grams per mole and you have 2396 grams needed per liter. This gives 1540 grams of solution. Therefore the mole is a unit for that physical quantity. This quantity is sometimes referred to as the chemical amount.
 Source: youtube.com
Source: youtube.com
These are two common ways to think about what the concentration 1 ppm means. This quantity is sometimes referred to as the chemical amount. A 1M solution would consist of 3423 grams sucrose in one liter final volume. N_t n_a. 1 millimole Fe2O3 15969 1000 01597 grams 15969 milligrams.
 Source: study.com
Source: study.com
Mole fraction chi the Greek letter chi is the number of moles of a given component of a mixture divided by the total number of moles in the mixture. This gives 1540 grams of solution. In 2011 the measurement was refined to 602214078 000000018 10 23 mol 1. Therefore the mole is a unit for that physical quantity. I think unfortunately the terminology gram-mole has been used as the mass in grams of a mole of things based on number of carbon atoms in 12 grams of carbon-12 whereas something like the pound-mole would be the mass in pounds of a mole of things based on number of carbon.
 Source: wikihow.com
Source: wikihow.com
Use this page to learn how to convert between moles NO and gram. This property simplifies many chemical computations. Since the unified atomic mass unit symbol. Add the weight of each atom in the compound. The mole was defined in such a way that the molar mass of a compound in gmol is numerically equal for all practical purposes to the average mass of one molecule in daltons.
 Source: slideplayer.com
Source: slideplayer.com
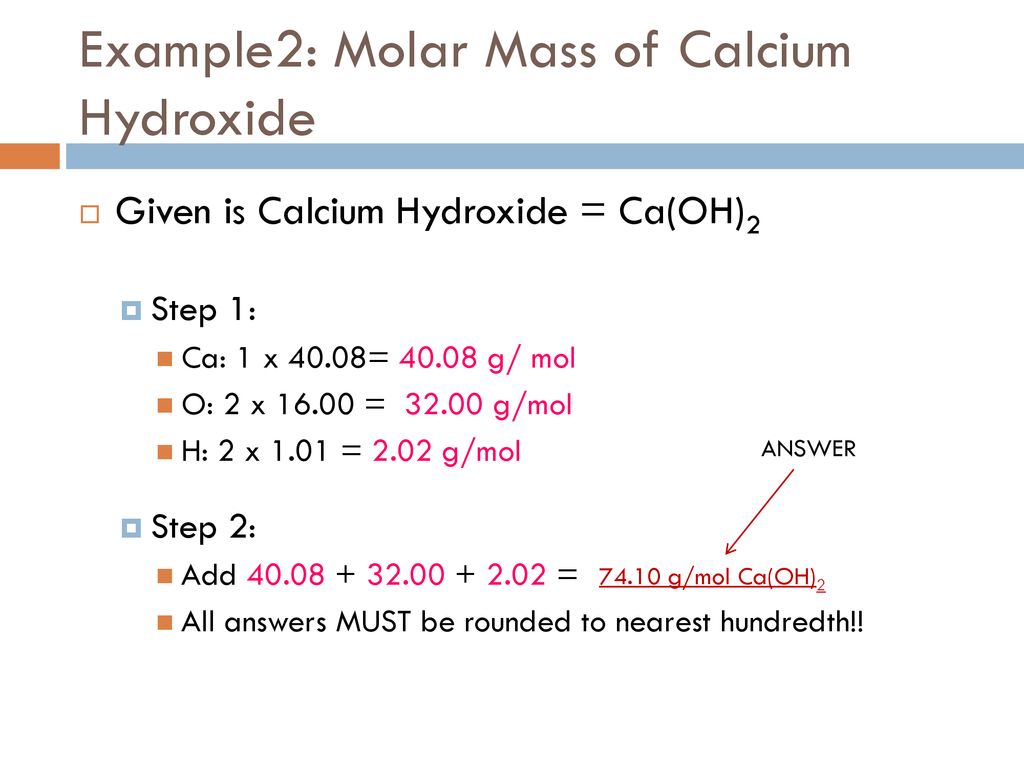
A concentration of 70 mM is the same as 007 moles per liter. The unit of molar mass is gramsmole. Since the unified atomic mass unit symbol. I think unfortunately the terminology gram-mole has been used as the mass in grams of a mole of things based on number of carbon atoms in 12 grams of carbon-12 whereas something like the pound-mole would be the mass in pounds of a mole of things based on number of carbon. It is also sometimes written as molLs but the double division is ambiguous and should be avoided unless parentheses are used.
 Source: study.com
Source: study.com
The molar massmolecular weight is actually the sum of the total mass in grams of the atoms present to make up a molecule per mole. To make 200 milliliters of your solution multiply gramsliter by liters needed. The unit of molar mass is gramsmole. In 2011 the measurement was refined to 602214078 000000018 10 23 mol 1. 25 moles X 9809 gmol 245225 grams of sulfuric acid.
 Source: pinterest.com
Source: pinterest.com
The expression 1 ppm means a given solute exists at a concentration of one part per million parts of the solution. The gram per cubic centimetre is a unit of density in the CGS system commonly used in chemistry defined as mass in grams divided by volume in cubic centimetres. Gram molecular mass may be reported in grams or grams per mole gmol. 1 Gram-mole g-mol 1 000 Millimole mmol - Measurement calculator that can be. The molar mass also known as molecular weight is the sum of the total mass in grams of all the atoms that make up a mole of a particular molecule.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title what does grams per mol mean by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






