Your What does 2 moles per liter mean images are available. What does 2 moles per liter mean are a topic that is being searched for and liked by netizens today. You can Find and Download the What does 2 moles per liter mean files here. Find and Download all free photos.
If you’re searching for what does 2 moles per liter mean images information related to the what does 2 moles per liter mean topic, you have pay a visit to the ideal site. Our site always gives you suggestions for downloading the maximum quality video and image content, please kindly hunt and locate more informative video content and graphics that fit your interests.
What Does 2 Moles Per Liter Mean. Answer 1 of 2. What Does Molar Solution Mean. Moles mol Molarity M x Volume L 05 x 2. The kilogram-mole is the most uninteresting thing in chemistry.
 Two Moles Per Liter Greeting Card By Socks317 Redbubble From redbubble.com
Two Moles Per Liter Greeting Card By Socks317 Redbubble From redbubble.com
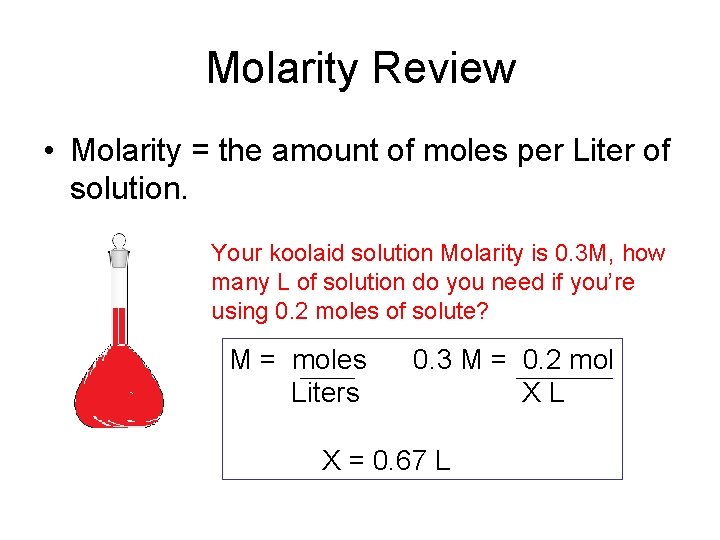
Molarity M indicates the number of moles of solute per liter of solution molesLiter and is one of the most common units used to measure the concentration of a solution. Liters of water 250 ml 1 L1000 ml liters of water 025 L. Once we know how many moles of AgBr dissolve in a liter of water we can calculate the solubility in grams per liter. Ag 2 50 x 10-13. The solvent may be a gas or liquid but is commonly a. One liter of a 005 N solution is the same as one liter of a 005 M solution of NaOH.
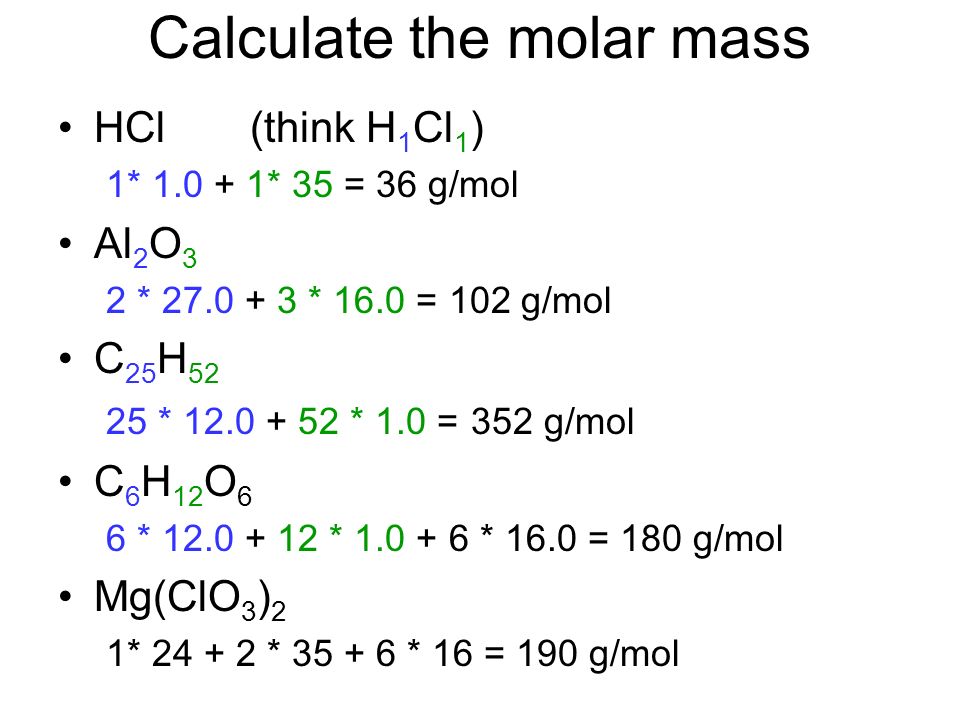
For NaCl the molar mass is 5844 gmol.
The moles per liter of solution are called molarity or molar concentration. If one mole of solute is dissolved in 1 kg of. Spicy sauce often containing chocolate 4. What is the molarity of a solution that contains 000372 moles hydrochloric acid in 239 x 10-2 liters of solution. The molar mass of sulfuric acid is 9809 gmol. We calculate that we will have a 005 M solution which is consistent with our expectations considering we diluted 25 mL of a concentrated solution to 2500 mL.
 Source: ifunny.co
Source: ifunny.co
First you must calculate the number of moles in this solution by rearranging the equation. Now we can use the rearranged equation. A liter is slightly larger than a quart. The molar mass of sulfuric acid is 9809 gmol. Molarity M indicates the number of moles of solute per liter of solution molesLiter and is one of the most common units used to measure the concentration of a solution.
 Source: amazon.com
Source: amazon.com
A solution with a concentration of 1 molL is said to be 1 molar commonly designated as 1 M. Spicy sauce often containing chocolate 4. Answer 1 of 2. Taking the square root of both sides of this equation gives the equilibrium concentrations of the Ag and Br-ions. Take 007 molesliter times 3423 grams per mole and you have 2396 grams needed per liter.
 Source: redbubble.com
Source: redbubble.com
Mass g No. Click to see full answer. In other words the solution has a concentration of 1 molL or a molarity of 1 1M. MOLE noun The noun MOLE has 6 senses. A small congenital pigmented spot on the skin.
 Source: goodreads.com
Source: goodreads.com
Molarity of solution mol KCL water. For NaCl the molar mass is 5844 gmol. What does n mm mean. A milliliter is a unit of fluid volume equal to one-thousandth of a liter. N mM n is the amount of substance in moles mol.
 Source: fashionloveshirts.com
Source: fashionloveshirts.com
The basic unit of amount of substance adopted under the Systeme International dUnites 2. 25M means 25 moles of sulfuric acid per one liter of solution. The solubility of AgBr in water is only 000013 gram per. Mass g No. Moles mol Molarity M x Volume L 05 x 2.
 Source: redbubble.com
Source: redbubble.com
Remember the volume refers to liters of solution not liters of water added to prepare the solution. Answer 1 of 2. Notice that all of the units for volume have been converted to liters. Liters of water 250 ml 1 L1000 ml liters of water 025 L. A 1M solution would consist of 3423 grams sucrose in one liter final volume.
 Source: slidetodoc.com
Source: slidetodoc.com
Taking the square root of both sides of this equation gives the equilibrium concentrations of the Ag and Br-ions. The solubility of AgBr in water is only 000013 gram per. So you will need 005 moles of NaOH for on liter. Molarity M indicates the number of moles of solute per liter of solution molesLiter and is one of the most common units used to measure the concentration of a solution. That is equal to about 0163 pounds of CO2.
 Source: slideplayer.com
Source: slideplayer.com
A liter is slightly larger than a quart. M is the mass of the substance in grams g. A nanomolar nM is the decimal fraction of a molar which is the common non-SI unit of molar concentration. If one mole of solute is dissolved in 1 kg of. The basic unit of amount of substance adopted under the Systeme International dUnites 2.
 Source: pinterest.com
Source: pinterest.com
A solution with a concentration of 1 molL is said to be 1 molar commonly designated as 1 M. Remember the volume refers to liters of solution not liters of water added to prepare the solution. Moles mol Molarity M x Volume L 05 x 2. A 1M solution would consist of 3423 grams sucrose in one liter final volume. MOLE noun The noun MOLE has 6 senses.
 Source: amazon.com
Source: amazon.com
For example 2-molar 2 M solution contains 2 moles of a certain substance in one liter of a liquid or gaseous mixture. Molarity of solution mol KCL water. A nanomolar nM is the decimal fraction of a molar which is the common non-SI unit of molar concentration. Convert 25 moles to grams. Now we can use the rearranged equation.
 Source: goodreads.com
Source: goodreads.com
We calculate that we will have a 005 M solution which is consistent with our expectations considering we diluted 25 mL of a concentrated solution to 2500 mL. To make 200 milliliters of your solution multiply gramsliter by liters needed. 25M means 25 moles of sulfuric acid per one liter of solution. Now we can use the rearranged equation. The basic unit of amount of substance adopted under the Systeme International dUnites 2.
 Source: redbubble.com
Source: redbubble.com
Ag 2 50 x 10-13. Molarity of solution mol KCL water. In chemistry the most commonly used unit for molarity is the number of moles per litre having the unit symbol molL. If one mole of solute is dissolved in 1 kg of. Finally youre ready to determine molarity.
 Source: pinterest.com
Source: pinterest.com
First you must calculate the number of moles in this solution by rearranging the equation. That is equal to about 0163 pounds of CO2. Calculate the molarity of a flask contains 154 moles potassium sulfate in 125 ml of. In this case you get 2 moles of hydroxide for each mole of CaOH2 and the normality of a 1 M solution of CaOH2 is 2 with respect to hydroxide. MOLE noun The noun MOLE has 6 senses.
 Source: amazon.com
Source: amazon.com
A 6 molar 6 M solution of H 2 SO 4 refers to a solution with six moles of sulfuric acid per liter of solution. Units per milliliter UmL The results of some medical tests are reported in units per milliliter UmLA unit is an arbitrary amount agreed upon by scientists and doctors. We calculate that we will have a 005 M solution which is consistent with our expectations considering we diluted 25 mL of a concentrated solution to 2500 mL. Now we can use the rearranged equation. If one mole of solute is dissolved in 1 liter of solution then the molarity of the solution is said to be 1M.
 Source: redbubble.com
Source: redbubble.com
MOLE noun The noun MOLE has 6 senses. A solution with a concentration of 1 molL is said to be 1 molar commonly designated as 1 M. Now we can use the rearranged equation. A molar solution is defined as an aqueous solution that contains 1 mole gram-molecular weight of a compound dissolved in 1 liter of a solution. The units are mole per liter.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title what does 2 moles per liter mean by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.