Your Video how to convert moles to grams and atoms of a compound images are ready. Video how to convert moles to grams and atoms of a compound are a topic that is being searched for and liked by netizens now. You can Download the Video how to convert moles to grams and atoms of a compound files here. Get all free images.
If you’re searching for video how to convert moles to grams and atoms of a compound pictures information related to the video how to convert moles to grams and atoms of a compound keyword, you have pay a visit to the ideal blog. Our site always gives you suggestions for viewing the maximum quality video and image content, please kindly surf and locate more informative video content and graphics that match your interests.
Video How To Convert Moles To Grams And Atoms Of A Compound. Avogadros number is a very important relationship to remember. 12 g CH 4. The units for molar mass are grams per mole or gmol. Molecular weight of I or mol The SI base unit for amount of substance is the mole.
 Hubert Hudson Fold Frog Convert Moles To Grams Calculator Uctsc Org From uctsc.org
Hubert Hudson Fold Frog Convert Moles To Grams Calculator Uctsc Org From uctsc.org
How many moles Be in 1 grams. 100 mole of any element has a mass numerically equal to its atomic mass in grams and contains 60221023 particles. B moles x 60221023 atoms 1 mole C atoms. Multiply the moles of the given substance molar mass. How do I convert moles to grams easily. Now its time to look at the specific experiment you are studying.
You can view more details on each measurement unit.
When converting grams to moles we need to know two things. 1 mole is equal to 1 moles Be or 9012182 grams. The mole abbreviated mol is the SI measure of quantity of a chemical entity such as atoms electrons or protons. Fracmolar mass1 mole. The molar mass of a compound or element allows conversion between mass in grams and the number of moles. For example say you started with 40 grams of oxygen.
 Source: youtube.com
Source: youtube.com
B moles x 60221023 atoms 1 mole C atoms. For example say you started with 40 grams of oxygen. Divide the variety of grams of the compound NaOH by the molecular weight and as. 1 mole 60221023 6022 10 23 atoms molecules protons etc. Dimensional Analysis Atoms To Grams Youtube.
 Source: youtube.com
Source: youtube.com
The equation to convert moles to atoms is as follows. Finally divide the number of grams of the compound by the molar mass of the compound to find the number of moles. Look at the conversion map. We assume you are converting between moles Be and gram. Divide the variety of grams of the compound NaOH by the molecular weight and as.
 Source: socratic.org
Source: socratic.org
How do we set up the problem. The molar mass of a compound or element allows conversion between mass in grams and the number of moles. How to Convert Moles to Grams. The mole abbreviated mol is the SI measure of quantity of a chemical entity such as atoms electrons or protons. How many moles Be in 1 grams.
 Source: youtube.com
Source: youtube.com
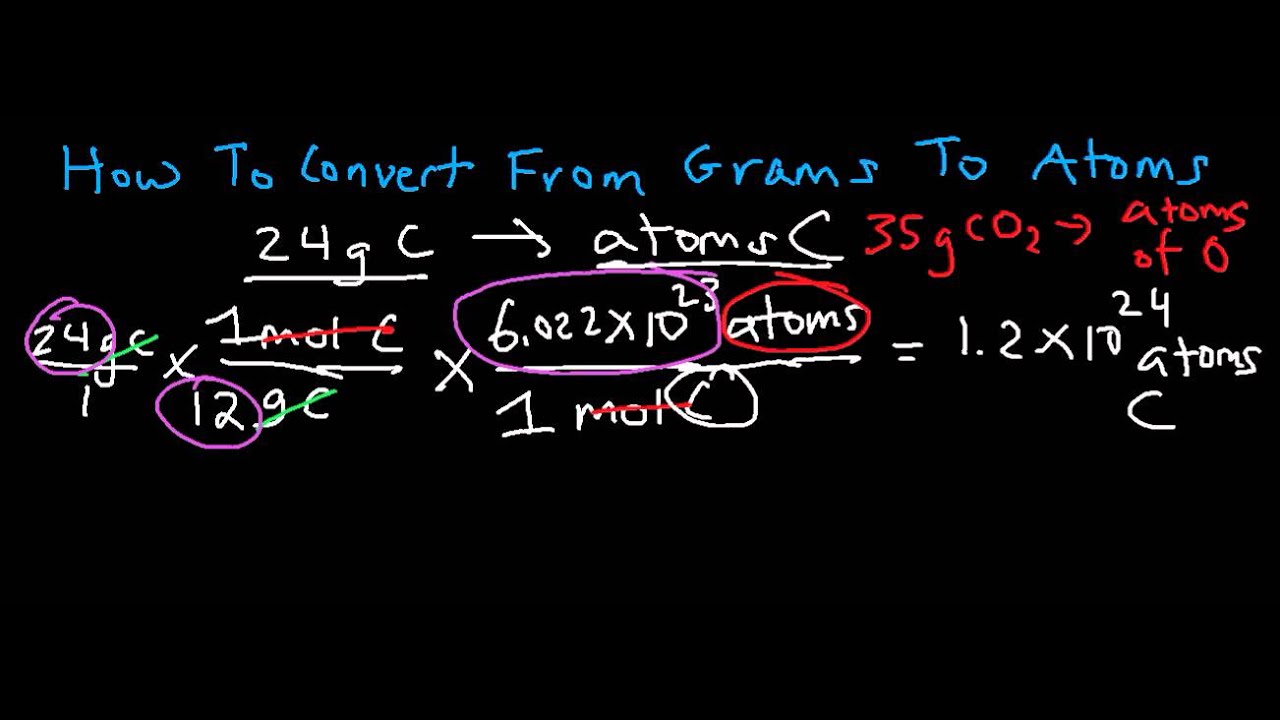
So 1 mol contains 602210 23 elementary entities of the substance. The number of grams of the chemical. To convert from atoms to moles divide the atom amount by Avogadros number or multiply by its reciprocal. Avogadros number is a very important relationship to remember. This chemistry video tutorial explains the conversion process of atoms to grams which is a typical step in common dimensional analysis stoichiometry problems.
 Source: youtube.com
Source: youtube.com
100 mole of any element has a mass numerically equal to its atomic mass in grams and contains 60221023 particles. The answer is 12690447. Fracmolar mass1 mole. The answer is 011096091934229. 12 g CH 4.
 Source: khanacademy.org
Source: khanacademy.org
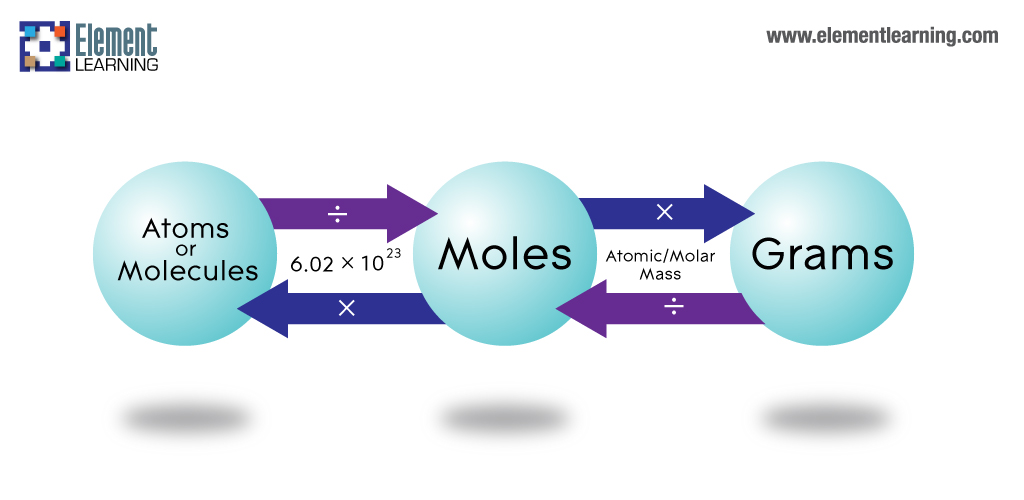
Look at the conversion map. To convert moles to grams use this conversion factor. In other words you take the number of moles of a substance B and then multiply it by Avogadros number divided by one mole. Look at the conversion map. 12 g CH 4.
 Source: uctsc.org
Source: uctsc.org
1 mole is equal to 1 moles Be or 9012182 grams. Divide the variety of grams of the compound NaOH by the molecular weight and as. We assume you are converting between grams I and mole. 1 mole 60221023 6022 10 23 atoms molecules protons etc. How many moles Be in 1 grams.
 Source: youtube.com
Source: youtube.com
Look at the conversion map. How many grams I in 1 mol. Avogadros number is a very important relationship to remember. On this video I exploit molar mass to transform from grams to moles and from moles to grams000 Intro051 Grams to Moles example134 Miles to Grams instance Thi. Molecular weight of Be or grams The SI base unit for amount of substance is the mole.
 Source: youtube.com
Source: youtube.com
Write down the amounts of each reactant in grams. 1 mole 60221023 6022 10 23 atoms molecules protons etc. Look at the conversion map. We pass through 3 arrows when we go from Grams Moles Molecules Atoms. How many atoms are there in six moles of iron.
 Source: youtube.com
Source: youtube.com
The right way to convert to grams to moles. The answer is 12690447. How many moles Be in 1 grams. Divide this value by that compounds molar mass to convert the amount to moles. Changing Moles to Atoms Given a identified variety of moles x one can discover the variety of atoms y on this molar amount by multiplying it by Avogadros quantity.
 Source: study.com
Source: study.com
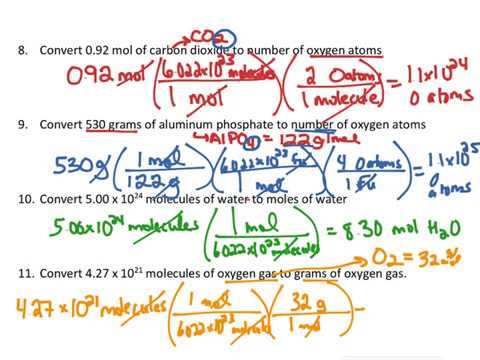
You can view more details on each measurement unit. Multiply the moles of the given substance molar mass. Converting between grams of a compound and grams of a constituent element Determine the mass of oxygen in a 58 g of NaHCO3 58 g NaCHO3 1 mol NaCHO3 8401 NaCHO3 4800 mol O 1 mol NaCHO3 1600 g O 1 mol I got 53 but the answer is 33 g O Help what am I doing wrong. You can view more details on each measurement unit. 12 g CH 4.
 Source: youtube.com
Source: youtube.com
1 mole 60221023 6022 10 23 atoms molecules protons etc. Calculating And Converting Moles and Atoms. How to Convert Moles to Grams. Now its time to look at the specific experiment you are studying. In other words you take the number of moles of a substance B and then multiply it by Avogadros number divided by one mole.
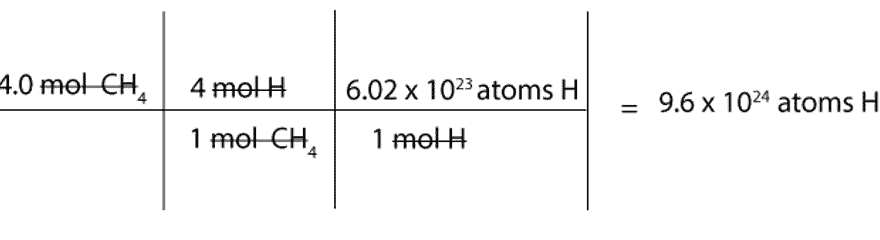 Source: masterconceptsinchemistry.com
Source: masterconceptsinchemistry.com
To convert moles to grams easily it is suggested to use the best online calculator provided here. A gramg is a metric unit of mass and was formally defined as the absolute weight of a volume of pure water equal to the cube of the hundredth part of a meter and at the temperature of melting ice. Converting between grams of a compound and grams of a constituent element Determine the mass of oxygen in a 58 g of NaHCO3 58 g NaCHO3 1 mol NaCHO3 8401 NaCHO3 4800 mol O 1 mol NaCHO3 1600 g O 1 mol I got 53 but the answer is 33 g O Help what am I doing wrong. This chemistry video tutorial explains find out how to convert the unit grams to moles which is a typical conversion step for a lot of stoichiometry questions. 3 arrows 3 conversion.
 Source: youtube.com
Source: youtube.com
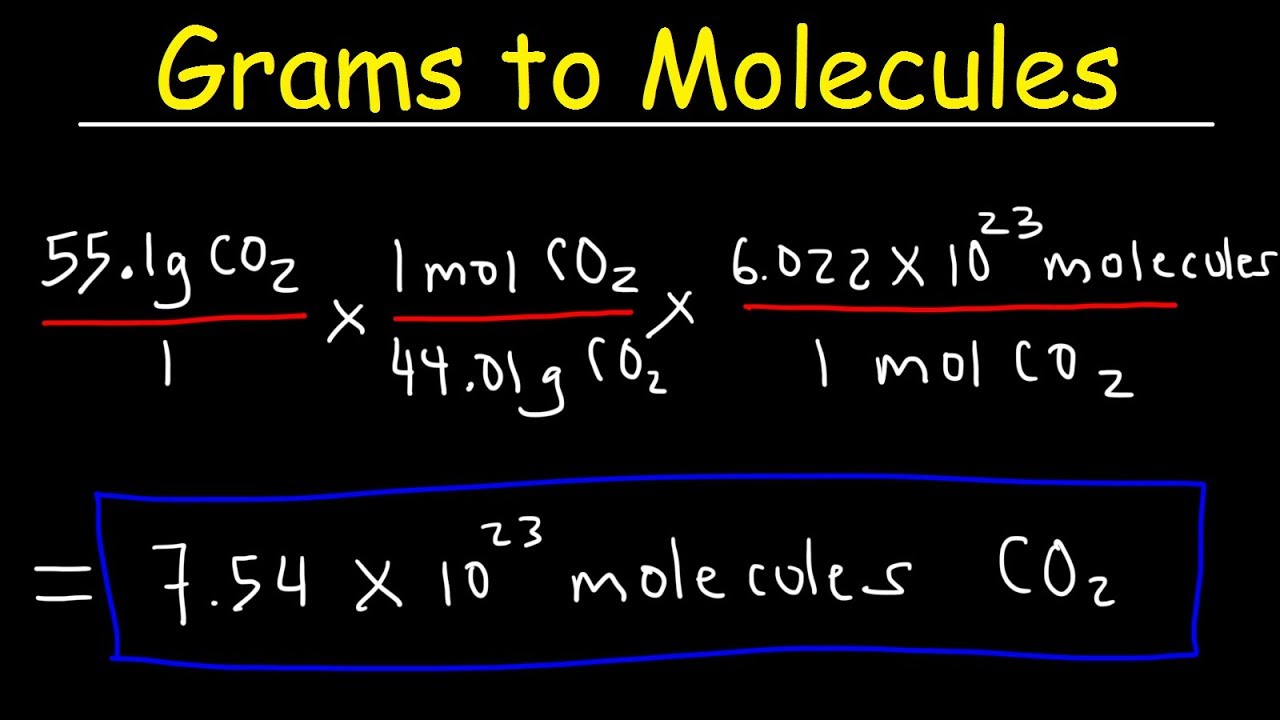
This chemistry video tutorial explains find out how to convert the unit grams to moles which is a typical conversion step for a lot of stoichiometry questions. The mole abbreviated mol is the SI measure of quantity of a chemical entity such as atoms electrons or protons. Molecular weight of I or mol The SI base unit for amount of substance is the mole. To convert from moles to atoms multiply the molar amount by Avogadros number. FAQs on Moles to Grams Converter.
 Source: onlinemathlearning.com
Source: onlinemathlearning.com
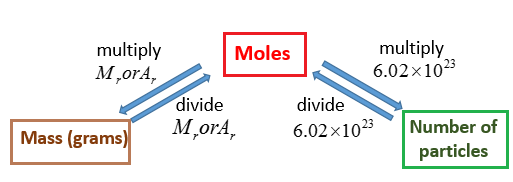
Divide the variety of grams of the compound NaOH by the molecular weight and as. First box is info given next 3 boxes are the 3 conversion last box fifth box is what the question asked for. To convert grams to moles start by multiplying the number of atoms by the atomic weight for each element in the compoundThen add all of your answers together to find the molar mass of the compound. How many atoms are there in six moles of iron. How do we set up the problem.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title video how to convert moles to grams and atoms of a compound by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






