Your Stoichiometry how to convert grams to moles images are available in this site. Stoichiometry how to convert grams to moles are a topic that is being searched for and liked by netizens now. You can Get the Stoichiometry how to convert grams to moles files here. Download all free images.
If you’re searching for stoichiometry how to convert grams to moles images information linked to the stoichiometry how to convert grams to moles topic, you have come to the ideal blog. Our site frequently provides you with suggestions for refferencing the maximum quality video and picture content, please kindly search and find more informative video articles and graphics that fit your interests.
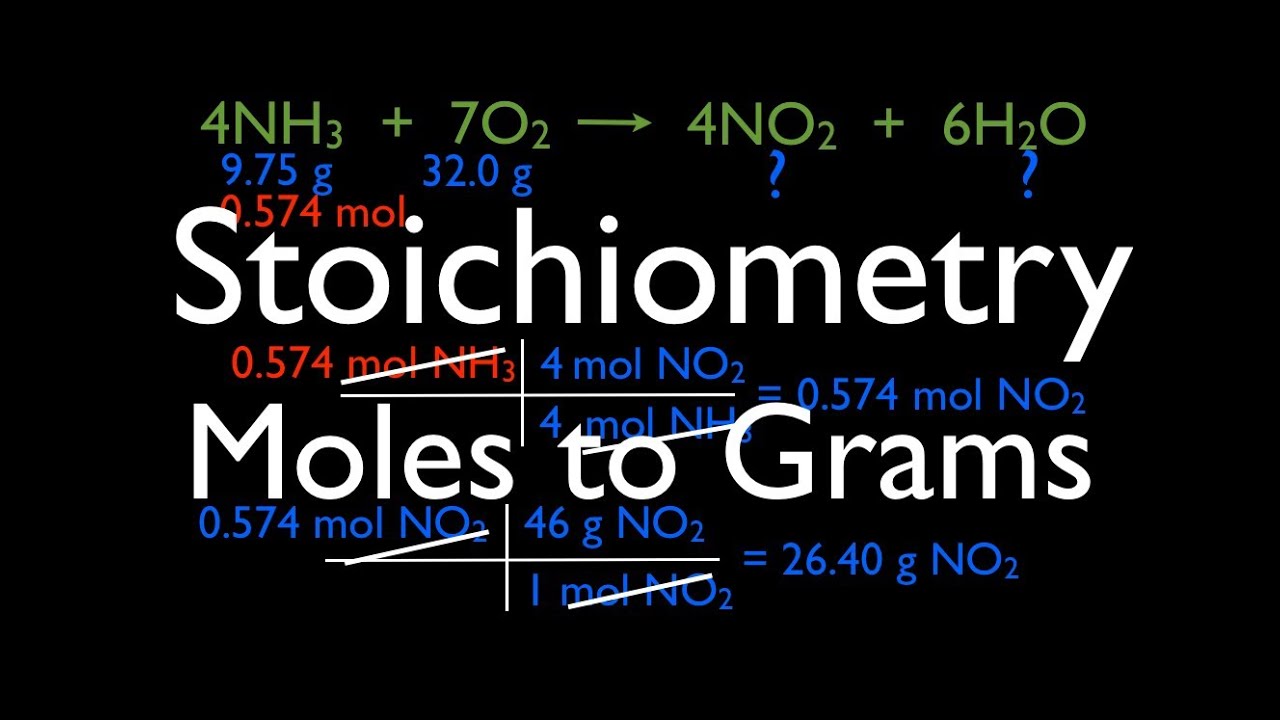
Stoichiometry How To Convert Grams To Moles. Convert 475 g C to moles of C 475 g C x 1 mol C 1201g C Simplify to get. 245 lmol 1 liter of oxygen is 1245 00408 mol conversion to mass. Now lets use equation 1 and multiply each side by 1 gram s as we can do in algebra. In chemistry mass is conserved.
 Reaction Stoichiometry Mole Method Calculations Coefficients In Balanced Equations Give The Ratio By Moles 2 C 4 H 10 13 O 2 8 Equations Method Chemistry From pinterest.com
Reaction Stoichiometry Mole Method Calculations Coefficients In Balanced Equations Give The Ratio By Moles 2 C 4 H 10 13 O 2 8 Equations Method Chemistry From pinterest.com
Gram to Gram Stoichiometry April 16 2020. O If given an amount in liters you should also be. Limiting Reagent Examples Al — 100 g 26982 gmol 037062 mol O 3 — 190 g 47997 gmol 039586 mol 3 Determine limiting reagent. High School Chemistry Lesson. 50 Stoichiometry Worksheet Answer Key In 2020 Persuasive. 539 2 h 2 g o 2 g 2 h 2 o g thus 2 mol of h 2 react with 1 mol of o 2 to produce 2 mol of h 2 o.
6 mol o 2 _____.
April 16 2020 ObjectiveLearning Target. Cancel out units and multiplyCheck to see i f you answer makes senseConvert 962 grams of h2o to molesConvert grams of each in mols of each element using atomic weights. Well learn how to convert back and forth between grams and moles. Calculation process for converting grams to moles using conversion factors for stoichiometry. 2 Convert grams to moles. Stoichiometry is all about relationships.
 Source: pinterest.com
Source: pinterest.com
Here we know that 1 mole of H 2 O is about 18 grams look at the GFM. Convert moles of the wanted substance to the desired units. April 16 2020 ObjectiveLearning Target. Stoichiometry practice problems balance the following equations first then answer the questionsSubstance in moles grams and concentration of substance in moll gl for conversion from mass to molarity divide the mass g or gl with molar mass relative awmwfw for conversion from molarity to mass multiply the molarity mol or. Calculation process for converting grams to moles using conversion factors for stoichiometry.
 Source: pinterest.com
Source: pinterest.com
Al to Al 2 O. Well learn how to convert back and forth between grams and moles. 245 lmol 1 liter of oxygen is 1245 00408 mol conversion to mass. For example to find the amount of nacl sodium chloride in 200 g one would do the following. How many grams of sodium chloride are required to make 032 moles of chlorine.
 Source: pinterest.com
Source: pinterest.com
This dimensional analysis video tuto. This dimensional analysis video tuto. How many grams of sodium chloride are required to make 032 moles of chlorine. A recipe for a parrillada calls for 3lbs of fajita 1lb of chicken and 2lb of sausage. Craig Beals explains the process of mole to mass stoichiometry in Chemistry.
 Source: pinterest.com
Source: pinterest.com
539 2 h 2 g o 2 g 2 h 2 o g thus 2 mol of h 2 react with 1 mol of o 2 to produce 2 mol of h 2 o. High School Chemistry Lesson. 50 Stoichiometry Worksheet Answer Key In 2020 Persuasive. This chemistry video tutorial explains how to convert moles to grams which is useful in typical stoichiometry problems. First a thinking through it approach that shows.
 Source: pinterest.com
Source: pinterest.com
Convert moles of the wanted substance to the desired units. A recipe for a parrillada calls for 3lbs of fajita 1lb of chicken and 2lb of sausage. 962 grams H 2 O x 1 mole H 2 O1802 grams 962 x 1 mole 1802 053 moles Check to see if your answer makes sense. Stoichiometry practice problems balance the following equations first then answer the questionsSubstance in moles grams and concentration of substance in moll gl for conversion from mass to molarity divide the mass g or gl with molar mass relative awmwfw for conversion from molarity to mass multiply the molarity mol or. Limiting Reagent Examples Al — 100 g 26982 gmol 037062 mol O 3 — 190 g 47997 gmol 039586 mol 3 Determine limiting reagent.
 Source: pinterest.com
Source: pinterest.com
We use these relationships to convert from one substanceunit to another. Remember that molar weight is given in gramsmole so you would need to divide the mass by the molar mass to get moles. Or in plain English two moles of hydrogen and one mole of oxygen react to produce two moles of water. Al — 037062 2 018531 O 3 — 039586 1 039586 Al is the limiting reagent 4 Determine moles of product formed. Gram to Gram Stoichiometry April 16 2020.
 Source: pinterest.com
Source: pinterest.com
Stoichiometry practice problems balance the following equations first then answer the questionsSubstance in moles grams and concentration of substance in moll gl for conversion from mass to molarity divide the mass g or gl with molar mass relative awmwfw for conversion from molarity to mass multiply the molarity mol or. Students will be able to convert between grams of the various substances in a chemical reaction. Stoichiometry practice problems balance the following equations first then answer the questionsSubstance in moles grams and concentration of substance in moll gl for conversion from mass to molarity divide the mass g or gl with molar mass relative awmwfw for conversion from molarity to mass multiply the molarity mol or. Craig Beals explains the process of mole to mass stoichiometry in Chemistry. Al — 037062 2 018531 O 3 — 039586 1 039586 Al is the limiting reagent 4 Determine moles of product formed.
 Source: pinterest.com
Source: pinterest.com
If youre seeing this message it means were having trouble loading external resources on our website. Al to Al 2 O. Gram to Gram Stoichiometry April 16 2020. In chemistry mass is conserved. Calculation process for converting grams to moles using conversion factors for stoichiometry.
 Source: co.pinterest.com
Source: co.pinterest.com
Practice converting moles to grams and from grams to moles when given the molecular weight. Specifically between the reactants and products in chemical reactions. In chemistry mass is conserved. 539 2 h 2 g o 2 g 2 h 2 o g thus 2 mol of h 2 react with 1 mol of o 2 to produce 2 mol of h 2 o. How To Do Stoichiometry Grams To Moles.
 Source: pinterest.com
Source: pinterest.com
High School Chemistry Lesson. 539 2 h 2 g o 2 g 2 h 2 o g thus 2 mol of h 2 react with 1 mol of o 2 to produce 2 mol of h 2 o. Now lets use equation 1 and multiply each side by 1 gram s as we can do in algebra. Gram to Gram Stoichiometry April 16 2020. If youre behind a web filter please make sure that the domains.
 Source: gr.pinterest.com
Source: gr.pinterest.com
If youre behind a web filter please make sure that the domains. For example to find the amount of nacl sodium chloride in 200 g one would do the following. 2 Convert grams to moles. Converting grams to moles. Cancel out units and multiplyCheck to see i f you answer makes senseConvert 962 grams of h2o to molesConvert grams of each in mols of each element using atomic weights.
 Source: pinterest.com
Source: pinterest.com
Specifically between the reactants and products in chemical reactions. This dimensional analysis video tuto. Specifically between the reactants and products in chemical reactions. Well learn how to convert back and forth between grams and moles. These steps will be used when the reactants are given in moles and the products.
 Source: pinterest.com
Source: pinterest.com
35 moles H 2 O x 1802 grams1 mole H 2 O 35 x 1802 grams 6307 grams Check to see if you answer makes sense. This chemistry video tutorial explains how to convert moles to grams which is useful in typical stoichiometry problems. Gram to Gram Stoichiometry April 16 2020. Convert 475 g C to moles of C 475 g C x 1 mol C 1201g C Simplify to get. 35 moles H 2 O x 1802 grams1 mole H 2 O 35 x 1802 grams 6307 grams Check to see if you answer makes sense.
 Source: pinterest.com
Source: pinterest.com
For example to find the amount of nacl sodium chloride in 200 g one would do the following. How many grams of sodium chloride are required to make 032 moles of chlorine. Craig Beals explains the process of mole to mass stoichiometry in Chemistry. For each example well do it two ways. First a thinking through it approach that shows.
 Source: pinterest.com
Source: pinterest.com
962 grams H 2 O x 1 mole H 2 O1802 grams 962 x 1 mole 1802 053 moles Check to see if your answer makes sense. This dimensional analysis video tuto. For example you may be given moles of A and need to convert to grams of B. April 16 2020 ObjectiveLearning Target. Convert 312 mol Na to grams Na 312 mol Na x 2299g Na 1 mol Na Simplify to get.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title stoichiometry how to convert grams to moles by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.