Your One mole of oxygen gas weighs approximately images are available in this site. One mole of oxygen gas weighs approximately are a topic that is being searched for and liked by netizens now. You can Find and Download the One mole of oxygen gas weighs approximately files here. Get all royalty-free photos.
If you’re searching for one mole of oxygen gas weighs approximately images information connected with to the one mole of oxygen gas weighs approximately interest, you have come to the ideal blog. Our website always provides you with hints for seeing the highest quality video and image content, please kindly search and locate more enlightening video content and graphics that fit your interests.
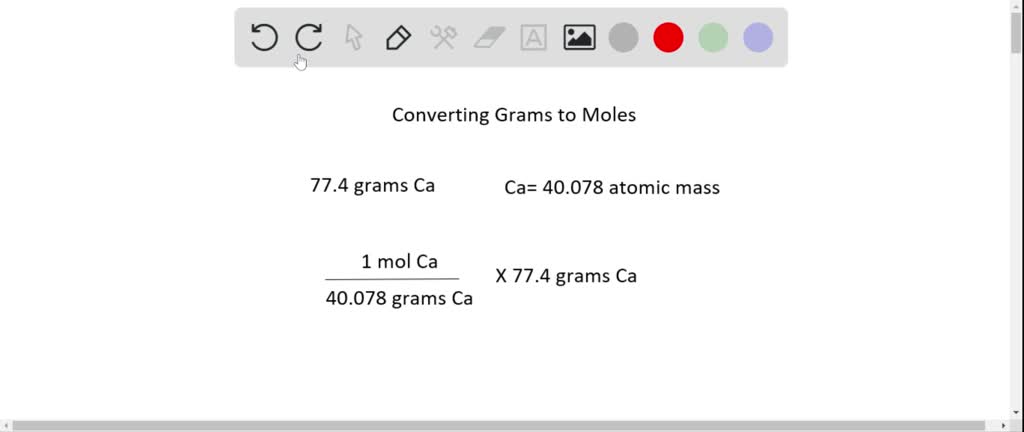
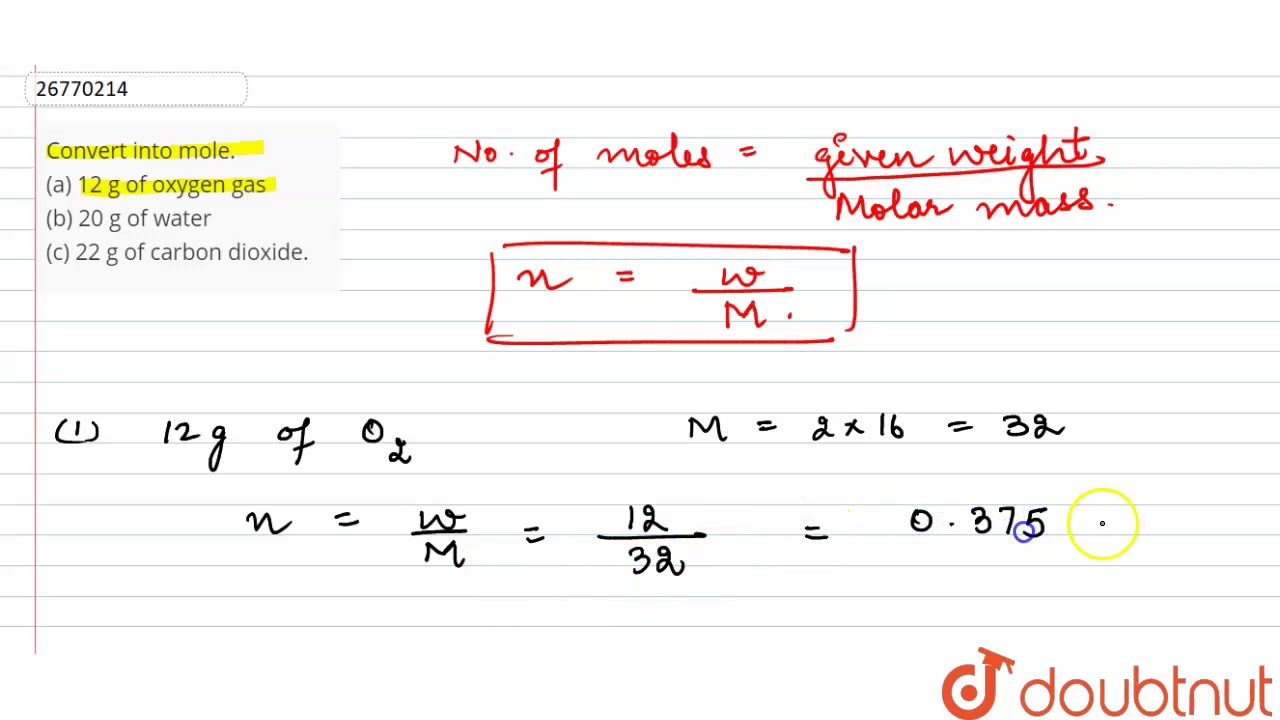
One Mole Of Oxygen Gas Weighs Approximately. Calculate the percent by volume of oxygen gas in the mixture if the diver has to submerge to a depth where the total pressure is 42 atm. Approximately how many moles are there in 340 grams of Na3PO4. 1 mole of oxygen gas O 2 weighs approximately 32g and 1 mole of hydrogen gas H 2 weighs approximately 2g. A 04 mole of iron.
 Identify The Correct Statement Regarding T Clutch Prep From clutchprep.com
Identify The Correct Statement Regarding T Clutch Prep From clutchprep.com
Since molar volume refers to the volume occupied by 1 mole youd get. Calculate the empirical formula and molecular formula of the phosphorus oxide given the molar mass is approximately 284 gmol. First convert the mass of oxygen to moles of oxygen. The same as the mass of one mole of the compound in grams. Multiply 555 cm times 2 cm and round off the answer. Skill 3-1 Calculate the molecular mass of a compound as the sum of the atomic masses of its elements.
The mass of an oxygen atom 16 amu.
The amount of space occupied by a mole of oxygen is then given by By Avogadros Law 224 liters of any other gas at STP will contain the same number of molecules as a mole of oxygen. Mass of O 2 molecule 2 16 32 amu. PbSO 4 2 LiNO 3 PbNO 3 4 Li 2SO 4. A 1000 g sample of red phosphorus powder was burned in air and reacted with oxygen gas to give 2291 g of a phosphorus oxide. TFThe correct answer for the product 2150 122 is 2623. Using the following equation.
 Source: youtube.com
Source: youtube.com
Convert 9500g of iron to number of atoms in the sample. Water for instance has a molecular weight of 18015 atomic mass units and one mole weighs approximately 18015 grams. Molecular mass H 2 O 2 x atomic mass of H atomic mass of O 21008 amu 1600 amu 1802 amu So one mole of water 6022 x 10 23 molecules has a mass of 1802 g. Briefly summarized the mass that constitutes one mole of a substance will be equal to its molecular weight. Substance in grams contains 1 mole 602 x 1023 units of that substance.

1 mole 602 x 1023 atoms 1 mole atomic mass g 5. One mole of iron A is heavier than one mole of lead Pb. Thus 224 liters of any gas at STP contains exactly one mole of the gas. TFThe correct answer for the product 2150 122 is 2623. Mass in grams of one mole of any element numerically equal to its atomic weight Molar mass of molecules can be determined from the chemical formula and molar masses of elements Each H 2O molecule contains 2 H atoms and 1 O atom Each mole of H 2O molecules contains 2 moles of H and 1 mole of O One mole of O atoms corresponds to 159994 g.

X 1600 you get the number of moles. Oxygen has a molar mass approximately 1600 g mol. Briefly summarized the mass that constitutes one mole of a substance will be equal to its molecular weight. 1 mole 602 x 1023 atoms 1 mole atomic mass g 5. Since molar volume refers to the volume occupied by 1 mole youd get.
 Source: youtube.com
Source: youtube.com
Mass of one molecule of oxygen 32 Avogadro constant 6023. Given the number of moles of iron 04 mol Number of iron atoms number of moles N A 04 602 10 23 2408 10 23 atoms b Oxygen gas O 2 is a molecular substance. Approximately how many moles are there in 340 grams of Na3PO4. 1 mole 602 x 1023 atoms 1 mole atomic mass g 5. 1 mole of NaCl weighs approximately 584.
 Source: clutchprep.com
Source: clutchprep.com
Avogadros number is 602210 23 molecules. Multiply 555 cm times 2 cm and round off the answer. Which one of the following does not represent 100 mol of the indicated substance. B is 770 g of iron. PbSO 4 2 LiNO 3 PbNO 3 4 Li 2SO 4.

Avogadros number is 602210 23 molecules. Therefore 1 mole of O 2 weighs 32 g and contains 602 x 1023 O 2 molecules. How many grams is equal to 348 x 1022 atoms of tin. For example the atomic weight of oxygen gas O 2 is 32. A gross of molecules is 144 molecules.
 Source: youtube.com
Source: youtube.com
One liter of oxygen at STP weighs 143 g and a mole of oxygen weighs 32 g. E None of the above. Avogadros number is a similar concept to that of a dozen or a gross. One mole of oxygen gas which has the formula O 2 has a mass of 32 g and contains 602 X 10 23 molecules of oxygen but 1204 X 10 23 2 X 602 X 10 23 atoms because each molecule of oxygen contains two oxygen atoms. For example the atomic weight of oxygen gas O 2 is 32.
 Source: youtube.com
Source: youtube.com
E None of the above. B 01 mole of oxygen gas. A 50 B 38 C 21 D 48 80. The same as the mass of one mole of the compound in grams. From the periodic table we see the atomic weight of hydrogen is 10079 and the atomic weight of oxygen is 159994.

A gross of molecules is 144 molecules. It is equal to 602210 23 mol -1 and is expressed as the symbol N A. Briefly summarized the mass that constitutes one mole of a substance will be equal to its molecular weight. The same as the mass of one mole of the compound in grams. Mass of one molecule of oxygen 32 Avogadro constant 6023.

Thus 224 liters of any gas at STP contains exactly one mole of the gas. 1 mole of oxygen gas O 2 weighs approximately 32g and 1 mole of hydrogen gas H 2 weighs approximately 2g. Mass of one molecule of oxygen 32 Avogadro constant 6023. Avogadros number is a similar concept to that of a dozen or a gross. C is 260 g of iron.
 Source: youtube.com
Source: youtube.com
Multiply 555 cm times 2 cm and round off the answer. Divide 2882 centimeters by 14575 seconds and round off the answer. Using the following equation. 1 mole of oxygen gas O 2 weighs approximately 32g and 1 mole of hydrogen gas H 2 weighs approximately 2g. P — 1000 g 3097 gmol 0032289 mol.
 Source: youtube.com
Source: youtube.com
1 mol O 2 0250 g O 2 x 781 x 10 3 mol O 2 320 g O 2. NT 1 mol273 K mol-K Notice there are four units on this constant. 1 mole 602 x 1023 atoms 1 mole atomic mass g 5. The amount of space occupied by a mole of oxygen is then given by By Avogadros Law 224 liters of any other gas at STP will contain the same number of molecules as a mole of oxygen. 0250 moles 270g 675 g Al 1 mole 7.
 Source: youtube.com
Source: youtube.com
We know the mass of 60221023 oxygen atoms which are present in 1 mole of an atom mass of 1 mole of an oxygen atom is 1599 gmol 16 gmol Therefore 1 mole of oxygen 16 g. NT 1 mol273 K mol-K Notice there are four units on this constant. Lets say you were given a temperature of 355 K and a pressure of 25 atm and asked to determine the gas molar volume at these conditions. 1 mol O 2 0250 g O 2 x 781 x 10 3 mol O 2 320 g O 2. 1 mole 602 x 1023 atoms 1 mole atomic mass g 5.
 Source: toppr.com
Source: toppr.com
A dozen molecules is 12 molecules. Water for instance has a molecular weight of 18015 atomic mass units and one mole weighs approximately 18015 grams. Skill 3-1 Calculate the molecular mass of a compound as the sum of the atomic masses of its elements. 08 grams of oxygen gas are lost from the tube. A gross of molecules is 144 molecules.
 Source: youtube.com
Source: youtube.com
One mole of iron A is heavier than one mole of lead Pb. Helium is mixed with oxygen gas for deep-sea divers. 1 Calculate moles of P and O. 1 mole of H2O 2 602214076 1023 of Hydrogen 602214076 1023 of Oxygen. Briefly summarized the mass that constitutes one mole of a substance will be equal to its molecular weight.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title one mole of oxygen gas weighs approximately by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.