Your One mole of oxygen gas weighs images are available. One mole of oxygen gas weighs are a topic that is being searched for and liked by netizens now. You can Find and Download the One mole of oxygen gas weighs files here. Get all free vectors.
If you’re searching for one mole of oxygen gas weighs pictures information linked to the one mole of oxygen gas weighs keyword, you have pay a visit to the ideal site. Our site frequently gives you suggestions for viewing the maximum quality video and image content, please kindly hunt and locate more enlightening video content and images that fit your interests.
One Mole Of Oxygen Gas Weighs. One mole of atoms of oxygen has a mass of 16 g as 16 is the atomic weight of oxygen and contains 602 X 10 23 atoms of oxygen. Note that rounding errors may occur so always check the results. The molar mass of oxygen found on the periodic table. How much does 0400 mole of 23 weigh.
 If 16 G Of Oxygen Contains 1 Mole Of Oxygen Atoms Calculate The Mass Of One Atom Of Oxygen Youtube From youtube.com
If 16 G Of Oxygen Contains 1 Mole Of Oxygen Atoms Calculate The Mass Of One Atom Of Oxygen Youtube From youtube.com
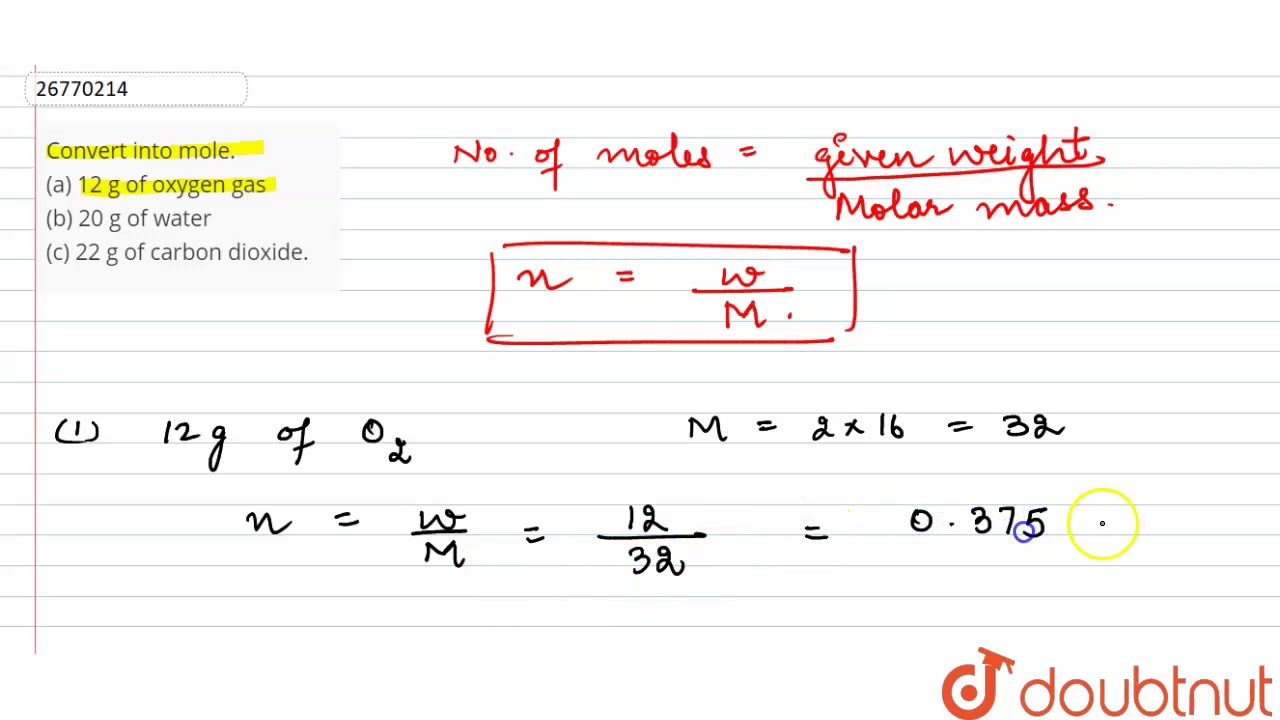
Here comes the key part. Number of particles in 1 mole N A 60231023mol1. One mole of oxygen gas weighs _______. Therefore the mass of O2 is 16 gmol x 2 mole oxygen 32 g for one mole oxygen gas. Mass of O 2 molecule 2 16 32 amu. 1 mole is equal to 1 moles Oxygen or 159994 grams.
And this weight is the sum of mass of electrons and protons present in.
Mass of one oxygen atom w NA 16 60231023. Mass of 1 mole oxygen atom W 16 g mol. And this weight is the sum of mass of electrons and protons present in. Calculate the molar mass of potassium chloride KCl. D 6023 x 1023 g. Some basic concepts of chemistry.
 Source: youtube.com
Source: youtube.com
So if we have the molar mass of the gas just divide it by 224 to get the density of that gas. The mass of one molecule of oxygen is. Molar mass of O2 gas 32 gmol. One mole of oxygen gas which has the formula O 2 has a mass of 32 g and contains 602 X 10 23 molecules of oxygen but 1204 X 10 23 2 X 602 X 10 23 atoms because each molecule of oxygen contains two oxygen atoms. Molecular weight of Oxygen or grams.

1 mole of H2 molecules 1g 1g 2g. Note that rounding errors may occur so always check the results. 1 mole is equal to 1 moles Oxygen or 159994 grams. How many atoms are present in 16 grams of oxygen. How much does 1 mole of gas weigh.
 Source: youtube.com
Source: youtube.com
Which weighs more a mole of nitrogen N 2 gas or a mole of oxygen O 2 gas. The mass of one molecule of oxygen is. How do you find the density of oxygen gas at STP. So if we have the molar mass of the gas just divide it by 224 to get the density of that gas. Therefore the mass of O2 is 16 gmol x 2 mole oxygen 32 g for one mole oxygen gas.
 Source: toppr.com
Source: toppr.com
O 2 is a diatomic molecule. 150 g MgOH₂ x 1 mole MgOH₂58326 g MgOH₂ x 6 moles H₂O3 moles MgOH₂ x 18016 g H₂O1 mole H₂O B 63 Find the mass of AlCl₃ that is produced when 250 grams of Al₂O₃ reacts with HCl according to. The mass of one molecule of oxygen is. The molecular formula for Oxygen is O. O 2 is a diatomic molecule.

150 g MgOH₂ x 1 mole MgOH₂58326 g MgOH₂ x 6 moles H₂O3 moles MgOH₂ x 18016 g H₂O1 mole H₂O B 63 Find the mass of AlCl₃ that is produced when 250 grams of Al₂O₃ reacts with HCl according to. This means 1 mole of hydrogen weighs 10079 grams and 1 mole of oxygen weighs. The mass of one molecule of oxygen is. 224 liters of H2 gas 2g. 1 mole of H2 molecules 6023 X 10 23 H2 molecule 224 liters of volume of H2 gas.

1 mole of H2 molecules 1g 1g 2g. Molar mass of O2 gas 32 gmol. The chemical formula for water is H20 which means that every molecule of water has 2 atoms of hydrogen H and one atom of oxygen O. Use this page to learn how to convert between moles Oxygen and gram. One mole helium Upvote7Downvote2ShareAnswer it4002602Accordingly what the molecular weight helium gas Helium atomic mass 4003 carbon 120107 oxygen 159994 Multiply the atomic mass number the molar mass constant defined.

O 2 is a diatomic molecule. What is the mass of exactly one mole of calcium acetate. When 4 moles of aluminum are allowed to react with an excess of chlorine gas Cl2 how many moles of aluminum chloride are produced. One mole H2 is 21008 gmol 2016 gram. This means 1 mole of hydrogen weighs 10079 grams and 1 mole of oxygen weighs.
 Source: youtube.com
Source: youtube.com
Mass of O 2 molecule 2 16 32 amu. D 6023 x 1023 g. Density of oxygen is equal to 1429 kgm³. One mole of atoms of oxygen has a mass of 16 g as 16 is the atomic weight of oxygen and contains 602 X 10 23 atoms of oxygen. B Oxygen gas O 2 is a molecular substance.
 Source: clutchprep.com
Source: clutchprep.com
How do you find the density of oxygen gas at STP. From the Periodic Table of Elements one sees that one mole of hydrogen atoms weighs 1 gram while one mole of oxygen atoms weighs 16 grams. Oxygen is one of the 7 diatomic elements so the formula is O2 for oxygen gas. The weight of one mole of octane molecules will be equal to the summation of the weights of eight carbon atoms at 12 gramsmole each from carbons mass number plus 18 hydrogen atoms at 1 grammole each. Therefore the mass of O2 is 16 gmol x 2 mole oxygen 32 g for one mole oxygen gas.

One mole helium Upvote7Downvote2ShareAnswer it4002602Accordingly what the molecular weight helium gas Helium atomic mass 4003 carbon 120107 oxygen 159994 Multiply the atomic mass number the molar mass constant defined. When 4 moles of aluminum are allowed to react with an excess of chlorine gas Cl2 how many moles of aluminum chloride are produced. Mass of 1 mole of oxygen atom is 16 gmol. One mole of oxygen gas weighs _______. Thus the number of molecules in 16 gm or 05 mole of oxygen gas are 05 x 6023 x 1023 3012 x 1023 molecules.

Use this page to learn how to convert between moles Oxygen and gram. Thus the number of molecules in 16 gm or 05 mole of oxygen gas are 05 x 6023 x 1023 3012 x 1023 molecules. Molecular mass is obtained by multiplying the atomic mass of an element with the number of atoms in the molecule and then adding the masses of all the elements in the molecule. For O 16 g mol. One mole helium Upvote7Downvote2ShareAnswer it4002602Accordingly what the molecular weight helium gas Helium atomic mass 4003 carbon 120107 oxygen 159994 Multiply the atomic mass number the molar mass constant defined.

Mass of O 2 molecule 2 16 32 amu. 150 g MgOH₂ x 1 mole MgOH₂58326 g MgOH₂ x 6 moles H₂O3 moles MgOH₂ x 18016 g H₂O1 mole H₂O B 63 Find the mass of AlCl₃ that is produced when 250 grams of Al₂O₃ reacts with HCl according to. And this weight is the sum of mass of electrons and protons present in. For O 16 g mol. So if we have the molar mass of the gas just divide it by 224 to get the density of that gas.
 Source: toppr.com
Source: toppr.com
1 mole of H2 molecules 1g 1g 2g. What is the mass of exactly one mole of calcium acetate. A mole of water molecules would be 2 moles of hydrogen atoms plus 1 mole of oxygen atoms. So 2 mole oxygen per mole oxygen gas. 1 mole is equal to 1 moles Oxygen or 159994 grams.
 Source: youtube.com
Source: youtube.com
One mole H2 is 21008 gmol 2016 gram. Density of oxygen is equal to 1429 kgm³. One mole helium Upvote7Downvote2ShareAnswer it4002602Accordingly what the molecular weight helium gas Helium atomic mass 4003 carbon 120107 oxygen 159994 Multiply the atomic mass number the molar mass constant defined. What is the mass of exactly one mole of calcium acetate. Number of particles in 1 mole N A 60231023mol1.
 Source: slideplayer.com
Source: slideplayer.com
For O 16 g mol. Number of particles in 1 mole N A 60231023mol1. At 0C 32F or 27315K at standard atmospheric pressure. For O 16 g mol. Thus the number of molecules in 16 gm or 05 mole of oxygen gas are 05 x 6023 x 1023 3012 x 1023 molecules.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title one mole of oxygen gas weighs by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.