Your One mole of oxygen gas is equal to images are ready. One mole of oxygen gas is equal to are a topic that is being searched for and liked by netizens now. You can Find and Download the One mole of oxygen gas is equal to files here. Find and Download all free images.
If you’re searching for one mole of oxygen gas is equal to pictures information related to the one mole of oxygen gas is equal to interest, you have pay a visit to the ideal site. Our website always provides you with suggestions for seeing the maximum quality video and image content, please kindly surf and find more enlightening video articles and images that fit your interests.
One Mole Of Oxygen Gas Is Equal To. The ideal gas law is PVnRT. Given the number of moles of iron 04 mol Number of iron atoms number of moles N A 04 602 10 23 2408 10 23 atoms b Oxygen gas O 2 is a molecular substance. 1 mole of O 2 is equal to N A Avogadro number of molecules of O 2 which means it has 6022 10 23 molecules of oxygen. Therefore 1 mole of O 2.
 How Many Moles Of Oxygen Gas Are Required For The Combustion Of One Mole Of 3 Methylpent 2 Ene Socratic From socratic.org
How Many Moles Of Oxygen Gas Are Required For The Combustion Of One Mole Of 3 Methylpent 2 Ene Socratic From socratic.org
So yes the temperatures of the gases will be equal. One mole of CO₂ gas contains 1 mole of carbon atoms and 2 moles of oxygen atoms. Atomic oxygen has one atom of oxygen its molar mass is 16gmol. Oxygen weighs 0001429 gram per cubic centimeter or 1429 kilogram per cubic meter ie. Given mass 12g. Hence 1 mole of oxygen gas is equal to molecular weight of oxygen as well as Avogadro number.
Given the number of moles of iron 04 mol Number of iron atoms number of moles N A 04 602 10 23 2408 10 23 atoms b Oxygen gas O 2 is a molecular substance.
So yes the temperatures of the gases will be equal. Some basic concepts of chemistry. I 6022 10 23 molecules of oxygen. Hence 1 mole of oxygen gas is equal to molecular weight of oxygen as well as Avogadro number. B 01 mole of oxygen gas. TRUE The molar mass of a compound in grams per mole is numerically equal to the formula mass of the compound in atomic mass units.
 Source: in.pinterest.com
Source: in.pinterest.com
Density of oxygen is equal to 1429 kgm³. 5032 15625 moles. Therefore chemists introduced the concept of molar volume. Atomic oxygen has one atom of oxygen its molar mass is 16gmol. Thus mass of 1 mole of oxygen is 32gm.

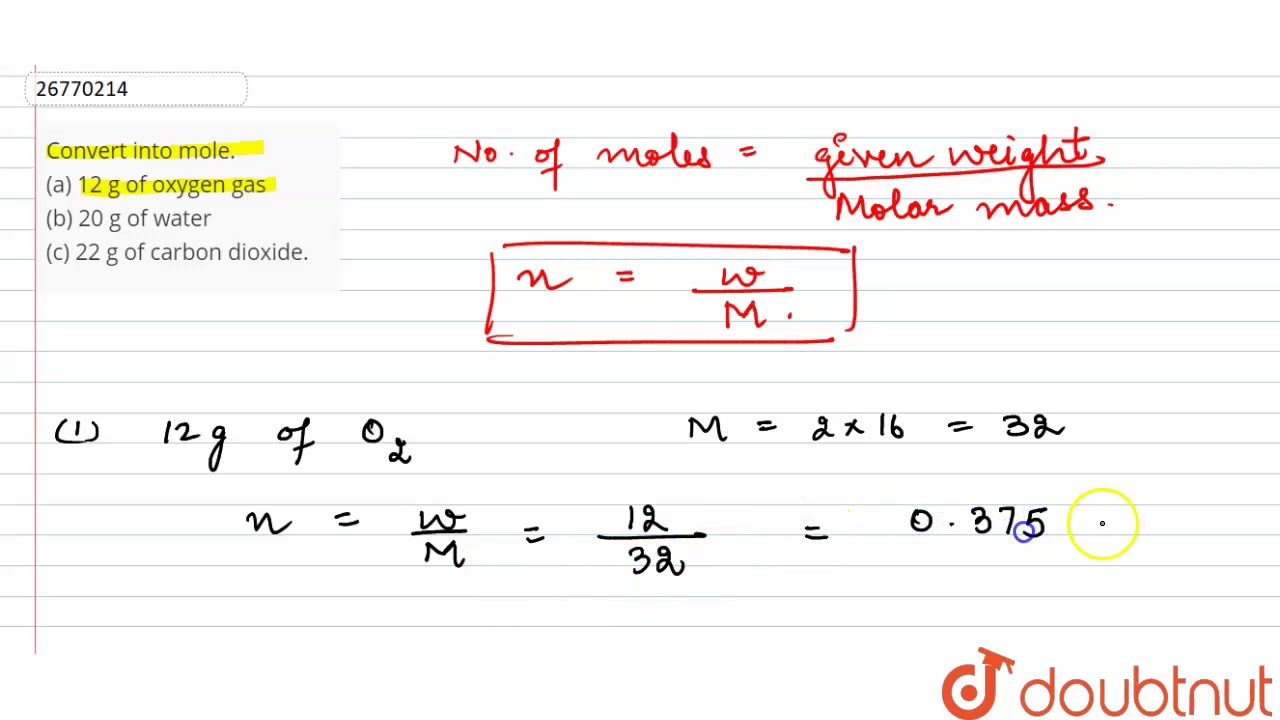
The molecular weight of oxygen O 2 16 2 32 g. Iii 16 g of oxygen. Number of particles number of moles Avogadros number. TRUE The molar mass of a compound in grams per mole is numerically equal to the formula mass of the compound in atomic mass units. From the formula of calculating moles Number of moles 12 32 0375 m o l e.
 Source: youtube.com
Source: youtube.com
B 01 mole of oxygen gas. Will be equal to equivalent oxygen. Thus mass of 1 mole of oxygen is 32gm. Iv 32 g of oxygen. Anyway 1 mole of oxygen gas contains 2 mole of oxygen atoms.

With the help of these two definitions lets see the whole solution. Use this page to learn how to convert between grams Oxygen and mole. The molecular weight of oxygen O 2 16 2 32 g. Oxygen weighs 0001429 gram per cubic centimeter or 1429 kilogram per cubic meter ie. A Iron Fe is an atomic substance.

Amole of nitrogen atoms is 14 grams and a mole of nitrogen molecules 28 gramsetc. Ii 6022 10 23 atoms of oxygen. Thus 4 moles of oxygen gas O2 would have a mass of 128 g. 1 10 -6. Iii 16 g of oxygen.
 Source: pinterest.com
Source: pinterest.com
Therefore chemists introduced the concept of molar volume. Note that rounding errors may occur so always check the results. Oxygen gas is composed of two homonuclear atoms therefore its molar mass is 162 32gmol. Iii 16 g of oxygen. 1 mole of oxygen gas at STP has 2 6022 10 23 atoms of.

Therefore 1 mole of O 2. Ii 6022 10 23 atoms of oxygen. Iv 32 g of oxygen. 1 000 000 000 000. O might also mean oxygen as the element without specifying its chemical form.
 Source: youtube.com
Source: youtube.com
The molecular weight of oxygen O 2 16 2 32 g. 1 mole of O_2 gas at STP6022xx1023 molecuels of O_2 Avogadro number 32g of O_2 Hence 1 mole of oxygen gas is equal to molecualr weight of oxygen as well as Avogadro number. O2 can only mean dioxygen gas. 1 mole of oxygen gas at STP has 2 6022 10 23 atoms of. Given to 3 digits and include the proper units grams.
 Source: youtube.com
Source: youtube.com
Oxygen gas is composed of two homonuclear atoms therefore its molar mass is 162 32gmol. The mole relates the atomic weight of an element in u to the mass of 1 mole of that element expressed in ___The mass of 1 mole of oxygen atoms is therefore equal to ___. Veronal and luminal are derivatives of barbituric acid. Iii 16 g of oxygen. It is the same as 1 dozen pairs of shoes and 2 dozen shoes.
 Source: youtube.com
Source: youtube.com
Atomic oxygen has one atom of oxygen its molar mass is 16gmol. 1 mole of O_2 gas at STP6022xx1023 molecuels of O_2 Avogadro number 32g of O_2 Hence 1 mole of oxygen gas is equal to molecualr weight of oxygen as well as Avogadro number. The molecular weight of oxygen O 2 16 2 32 g. I 6022 10 23 molecules of oxygen. Therefore 1 mole of oxygen gasoline is the same as molecular weight of oxygen in addition to Avogadro quantity.
 Source: socratic.org
Source: socratic.org
Therefore 12g of oxygen gas will have 1 32 12 0375 m o l e s. A 04 mole of iron. Therefore 1 mole of oxygen gasoline is the same as molecular weight of oxygen in addition to Avogadro quantity. We know that 32g of oxygen gas 1 mole. 1 grams Oxygen is equal to 0062502343837894 mole.

Is 8 gmequivalent because in a electrode reaction 4 moles of electrons deposit on one mole of O 2. The SI base unit for amount of substance is the mole. Atomic oxygen has one atom of oxygen its molar mass is 16gmol. Veronal and luminal are derivatives of barbituric acid. 1 mole of O_2 gas at STP6022xx1023 molecuels of O_2 Avogadro number 32g of O_2 Hence 1 mole of oxygen gas is equal to molecualr weight of oxygen as well as Avogadro number.
 Source: youtube.com
Source: youtube.com
Therefore 12g of oxygen gas will have 1 32 12 0375 m o l e s. O might also mean oxygen as the element without specifying its chemical form. Will be equal to equivalent oxygen. Since 1 mole of oxygen is equivalent to 32 g 4 moles of oxygen gas would be equivalent to 4 moles x 32 gmole 128 g. Veronal and luminal are derivatives of barbituric acid.
 Source: in.pinterest.com
Source: in.pinterest.com
Since there are 15 1022 molecules the number of moles of oxygen gas is equal to 15 1022 602 1023 00249 moles. So 01mol of O atoms will have a mass of 160116grams. Oxygen weighs 0001429 gram per cubic centimeter or 1429 kilogram per cubic meter ie. Some basic concepts of chemistry. Therefore 1 mole of oxygen gasoline is the same as molecular weight of oxygen in addition to Avogadro quantity.
 Source: youtube.com
Source: youtube.com
Iii 16 g of oxygen. 1 mole is equal to 1 moles Oxygen or 159994 grams. Note that rounding errors may occur so always check the results. Veronal and luminal are derivatives of barbituric acid. Given the number of moles of iron 04 mol Number of iron atoms number of moles N A 04 602 10 23 2408 10 23 atoms b Oxygen gas O 2 is a molecular substance.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title one mole of oxygen gas is equal to by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






