Your One mole of oxygen gas at stp is equal to images are available. One mole of oxygen gas at stp is equal to are a topic that is being searched for and liked by netizens now. You can Find and Download the One mole of oxygen gas at stp is equal to files here. Download all free images.
If you’re looking for one mole of oxygen gas at stp is equal to images information related to the one mole of oxygen gas at stp is equal to topic, you have pay a visit to the ideal blog. Our website frequently provides you with hints for downloading the highest quality video and picture content, please kindly hunt and locate more enlightening video content and images that match your interests.
One Mole Of Oxygen Gas At Stp Is Equal To. One mole of oxygen gas at STP is equal to. Remember that it was O 2 that was generated in this experiment so the molar mass we need is 3200 g. At STP one mole 6021023 representative particles of any gas occupies a volume of 224L figure below. Moles of O 2 02125 g O 2 1 mole3200 g O 2 6641 10 -3 moles O 2.
 One Mole Of Oxygen Gas At Stp Is Equal To From toppr.com
One Mole Of Oxygen Gas At Stp Is Equal To From toppr.com
It is equal to 224 litres of 22400 ml. Ad 1 mole of 0 2 gas at STP 6022 x 10 23 molecules of 0 2 Avogadro number 32 g of 0 2. Name the energy which arises due to motion of atoms or. Room conditions refer to the temperature of 25C and the pressure of 1 atmosphere. The most common example is the molar volume of a gas at STP Standard Temperature and Pressure which is equal to 224 L for 1 mole of any ideal gas at a temperature equal to 27315 K and a pressure equal to 100 atm. All four properties of gases ie P V n and T can be combined into a single equation which can.
Also calculate the percentage of empty space in one mole of the gas at STP.
Veronal and luminal are derivatives of barbituric acid. It is equal to 224 litres of 22400 ml. According to the mole concept. Some basic concepts of chemistry. One mole of oxygen gas at STP is equal to. 1 mole of any gas at stp is 224 liters.
 Source: youtube.com
Source: youtube.com
Room conditions refer to the temperature of 25C and the pressure of 1 atmosphere. Under room conditions one mole of any gas occupies 24 dm 3. Which substance has a molar mass of 142 g. Ad 1 mole of 0 2 gas at STP 6022 x 10 23 molecules of 0 2 Avogadro number 32 g of 0 2. Therefore one mol of either should have a volume of 224 L at STP.
 Source: youtube.com
Source: youtube.com
Iv 32 g of oxygen. 5032 15625 moles. What is equal to the number of particles in 24 grams of carbon-12. Standard Pressure 1 atm or 760 mm of mercury. Ii 6022 10 23 atoms of oxygen.
 Source: abetterchemtext.com
Source: abetterchemtext.com
Standard molar volume of a gas is the volume occupied by 1 mole of any gas at 273 K and 1 atm pressure STP. Where P is the pressure V the volume n is the number of moles of gas R is the gas constant and T is the temperature in degrees Kelvin. One mole of oxygen gasoline at stp is the same as. This means that one mole of any gas occupies the same volume at STP which is 224 dm 3. 1 mole of O2 gas at STP 60221023 molecules of O2 Avogadro number 32 g of O2.
 Source: meritnation.com
Source: meritnation.com
Iv 32 g of oxygen. Standard temperature and pressure STP are a useful set of benchmark conditions to compare other properties of gases. Therefore one mol of either should have a volume of 224 L at STP. Moles of O 2 02125 g O 2 1 mole3200 g O 2 6641 10 -3 moles O 2. Standard Pressure 1 atm or 760 mm of mercury.
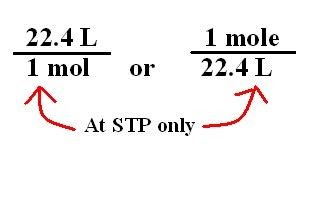 Source: socratic.org
Source: socratic.org
1 mole of any gas at stp is 224 liters. 5032 15625 moles. Hence 1 mole of oxygen gas is equal to molecular weight of oxygen as well as Avogadro number. What is equal to the number of particles in 24 grams of carbon-12. Molar Volume of Oxygen at STP.
 Source: toppr.com
Source: toppr.com
Asked Aug 18 2018 in Chemistry by Sagarmatha 545k points One mole of oxygen gas at STP is equal to _______. Hence 1 mole of oxygen gas is equal to molecular weight of oxygen as well as Avogadro number. Room conditions refer to the temperature of 25C and the pressure of 1 atmosphere. Under room conditions one mole of any gas occupies 24 dm 3. Standard Temperature 0 o C or 273 K.
 Source: toppr.com
Source: toppr.com
O is 16gmole so O2 is 32gmole. Moles of O 2 02125 g O 2 1 mole3200 g O 2 6641 10 -3 moles O 2. It is equal to 224 litres of 22400 ml. Standard temperature and pressure STP is defined as 0oC 27315K and 1atm pressure. Thus one mole of is equal to the molecular weight of oxygen and the Avogadro number.
 Source: answers.doubtnut.com
Source: answers.doubtnut.com
The most common example is the molar volume of a gas at STP Standard Temperature and Pressure which is equal to 224 L for 1 mole of any ideal gas at a temperature equal to 27315 K and a pressure equal to 100 atm. Standard temperature and pressure STP are a useful set of benchmark conditions to compare other properties of gases. All four properties of gases ie P V n and T can be combined into a single equation which can. Asked Aug 18 2018 in Chemistry by Sagarmatha 545k points One mole of oxygen gas at STP is equal to _______. The most common example is the molar volume of a gas at STP Standard Temperature and Pressure which is equal to 224 L for 1 mole of any ideal gas at a temperature equal to 27315 K and a pressure equal to 100 atm.

What is standard temperature and pressure for a gas. Also calculate the percentage of empty space in one mole of the gas at STP. Under room conditions one mole of any gas occupies 24 dm 3. The molar volume of a gas is the volume of one mole of a gas at STP. One mole of any gas remains constant and it is valued at 224l.
 Source: brainly.com
Source: brainly.com
Hence the volume of carbon dioxide occupied by it is 336 L. The most common example is the molar volume of a gas at STP Standard Temperature and Pressure which is equal to 224 L for 1 mole of any ideal gas at a temperature equal to 27315 K and a pressure equal to 100 atm. 5032 15625 moles. The ideal gas law can be used to determine densities of gases. Hence 1 mole of oxygen gas is equal to molecular weight of oxygen as well as Avogadro number.
 Source: toppr.com
Source: toppr.com
Iv 32 g of oxygen. Iv 32 g of oxygen. 5032 15625 moles. Which substance has a molar mass of 142 g. One mole of oxygen contains 60221023molecules or 260221023atoms of oxygen.
 Source: doubtnut.com
Source: doubtnut.com
One mole of oxygen contains 60221023molecules or 260221023atoms of oxygen. Some basic concepts of chemistry. 1 mole of oxygen gas at STP has 2 6022 10 23 atoms of oxygen. A 602 x 1023 iodine molecules 12. Former Principal of Scientific Consultancy ETGS.
 Source: toppr.com
Source: toppr.com
Its weight is equal to molecular weight which is 32gmol. What volume will 075 mole of oxygen gas occupy at STP. Also calculate the percentage of empty space in one mole of the gas at STP. Standard Pressure 1 atm or 760 mm of mercury. 1 mole of a gas occupies 224 Liters of a gas.

1 mole of oxygen gas at STP has 2 6022 10 23 atoms of oxygen. This means that one mole of any gas occupies the same volume at STP which is 224 dm 3. At STP one mole 6021023 representative particles of any gas occupies a volume of 224L figure below. At STP gases have a volume of 224 L per mole. One mole of oxygen contains 60221023molecules or 260221023atoms of oxygen.
 Source: youtube.com
Source: youtube.com
Hence 1 mole of oxygen gas is equal to molecular weight of oxygen as well as Avogadro number. 1 mole of any gas at stp is 224 liters. Standard temperature and pressure STP is defined as 0oC 27315K and 1atm pressure. I 6022 10 23 molecules of oxygen. Standard Pressure 1 atm or 760 mm of mercury.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title one mole of oxygen gas at stp is equal to by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






