Your One mole of carbon weighs 12 grams images are available. One mole of carbon weighs 12 grams are a topic that is being searched for and liked by netizens today. You can Find and Download the One mole of carbon weighs 12 grams files here. Download all royalty-free images.
If you’re looking for one mole of carbon weighs 12 grams images information related to the one mole of carbon weighs 12 grams interest, you have visit the right blog. Our site always provides you with hints for viewing the highest quality video and image content, please kindly search and locate more informative video content and images that match your interests.
One Mole Of Carbon Weighs 12 Grams. Atoms molecules ions is defined as a moleA mole of any substance is 602210 23 molecules. We know that one mole of any substance contains 60225X1023 particlesatoms molecules ions etc. One mole contains 60221023 molecules. 12 g 1 atom of carbon-12 That is 1 g 6022 X 1023 amu.
 Answered If One Mole Of Carbon Atoms Weighs 12 Grams What Is The Mass In Grams Of 1 Atom Of Carbon Brainly In From brainly.in
Answered If One Mole Of Carbon Atoms Weighs 12 Grams What Is The Mass In Grams Of 1 Atom Of Carbon Brainly In From brainly.in
One mole of anything is the same number of grams as the molecular mass. Hence the mass in grams of 1 atom of carbon is 19931023g. Hint A mole of carbon contains 6022 10 23 atoms so mass of 6022 10 23 atoms of carbon are 12 g. So 1 mole of carbon has mass 12 6022 1023 g 12 6022 10 23 g. So 1 atom of Carbon weighs 12 divided by 6023 10 to the power 23 g 12 10 to the power -23 623 g 199236261 10 to the power -23 g here -23 is minus 23. Therefore Mass of 6022 x 10 23 atoms of Carbon 12 g.
1 mole of carbon atoms 6022 x 10 23.
Hence the mass in grams of 1 atom of carbon is 19931023g. Hence mass of 1 carbon atom 12 6022 x 10 23. So find the mass of 1 atom of carbon using the given information. So we know that a mole of carbon contains 60221023 atoms. If one mole of carbon atoms weigh 12 gram what is the mass in gram of 1 atom of carbon. The value of the mole is equal to the number of atoms in exactly 12 grams of pure carbon-12.
 Source: toppr.com
Source: toppr.com
We can also relate the two mass scales grams and amu as follows. 6022 X 1023 atoms of carbon-12 X 12 amu 6022 X 1023 amu g. One mole of anything is the same number of grams as the molecular mass. In terms of mass a mole is equal to the weight of 12 grams of carbon-12. One mole of carbon-12 atoms has 602214076 10 23 atoms and a mass of 12 grams.
 Source: youtube.com
Source: youtube.com
Mass of one mole of carbon atom 12 g. Question From - NCERT Chemistry Class 9 Chapter 03 Question 013 ATOMS AND MOLECULES CBSE RBSE UP MP BIHAR BOARDQUESTION TEXT-If one mole of carbon at. We have given that one mole of carbon atom weighs 12 g. We know that Carbon has atomic mass equal to 12g I. Hint A mole of carbon contains 6022 10 23 atoms so mass of 6022 10 23 atoms of carbon are 12 g.
 Source: youtube.com
Source: youtube.com
One mole of carbon atom weighs 12g. Therefore we can write the mass of 60221023 atoms 12 g. So find the mass of 1 atom of carbon using the given information. Mass of 1 carbon atom 199 x 10-23g. 12 g 1 atom of carbon-12 That is 1 g 6022 X 1023 amu.
 Source: brainly.in
Source: brainly.in
The molecular mass of carbon atoms 12g. We know that Carbon has atomic mass equal to 12g I. 12 g 1 atom of carbon-12 That is 1 g 6022 X 1023 amu. One mole of anything is the same number of grams as the molecular mass. Atoms molecules ions is defined as a moleA mole of any substance is 602210 23 molecules.
 Source: youtube.com
Source: youtube.com
Mass of 1 atom of Carbon 12 6022 x 10 23. So 1 atom of Carbon weighs 12 divided by 6023 10 to the power 23 g 12 10 to the power -23 623 g 199236261 10 to the power -23 g here -23 is minus 23. Definition The molar mass of a given substance is defined as the mass of a sample divided by the moles of that substance in the sample. Hence mass of 1 carbon atom 12 6022 x 10 23. Solution 1 mole of C atoms 6022 10 23 atoms of C 12 g given.
 Source: geslab.net
Source: geslab.net
Hence mass of 60221023 atoms of carbon 12 g. One mole of carbon atoms weighs 12 g given ie mass of 1 mole of carbon atoms 12 g Then mass of 6022 10 23 number of carbon atoms 12 g Therefore mass of 1 atom of carbon 12 6022 10 23 19926 10 23 g. Mass of 1 carbon atom 199 x 10-23g. So find the mass of 1 atom of carbon using the given information. Mass of one mole of carbon atom 12 g.
 Source: toppr.com
Source: toppr.com
6022 x 1023 per mole. Hence mass of 60221023 atoms of carbon 12 g. We can also relate the two mass scales grams and amu as follows. One mole of carbon atom weighs 12g. Thus the absolute mass of 1 atoms of carbon is 199 1023 199 10 - 23 gram.
 Source: toppr.com
Source: toppr.com
1 mole of carbon atoms 6022 x 10 23. Mass of 1 atom of Carbon 12 6022 x 10 23. A mole of carbon contains 60221023 atoms. Atoms molecules ions is defined as a moleA mole of any substance is 602210 23 molecules. 1 mole of carbon-12 has 602 x 10 23 atoms.
 Source: brainly.in
Source: brainly.in
Hence mass of 60221023 atoms of carbon 12 g. 12 g 1 atom of carbon-12 That is 1 g 6022 X 1023 amu. One mole of carbon atoms weighs 12g Given ie mass of 1 mole of carbon atoms 12g. Now 6022 1023 6022 10 23 atoms of carbon have mass 12g 12 g. A mole is defined as exactly equal to 60221023 atoms.
 Source: brainly.in
Source: brainly.in
So find the mass of 1 atom of carbon using the given information. 6022 X 1023 atoms of carbon-12 X 12 amu 6022 X 1023 amu g. We have given that one mole of carbon atom weighs 12 g. So we know that a mole of carbon contains 60221023 atoms. Mass of 1 carbon atom 199 x 10-23g.
 Source: toppr.com
Source: toppr.com
Hence the correct option is A. One mole of anything is the same number of grams as the molecular mass. If one mole of carbon atoms weigh 12 gram what is the mass in gram of 1 atom of carbon. Solution 1 mole of C atoms 6022 10 23 atoms of C 12 g given. Second you need to know how many moles there are in 20 grams of carbon-12.
 Source: toppr.com
Source: toppr.com
Hence mass of 1 carbon atom 12 6022 x 10 23. Mass of 1 carbon atom 199 x 10-23g. By definition 12 grams of carbon-12 contain one mole or Avogadros number of carbon-12 atoms. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. 6022 x 1023 per mole.
 Source: scholr.com
Source: scholr.com
1 mole of carbon-12 has 602 x 10 23 atoms. Thus the absolute mass of 1 atoms of carbon is 199 1023 199 10 - 23 gram. If one mole of carbon atoms weighs. This means that the mass of 6022 1023 6022 10 23 atoms of carbon is 12 12 grams. By definition 12 grams of carbon-12 contain one mole or Avogadros number of carbon-12 atoms.
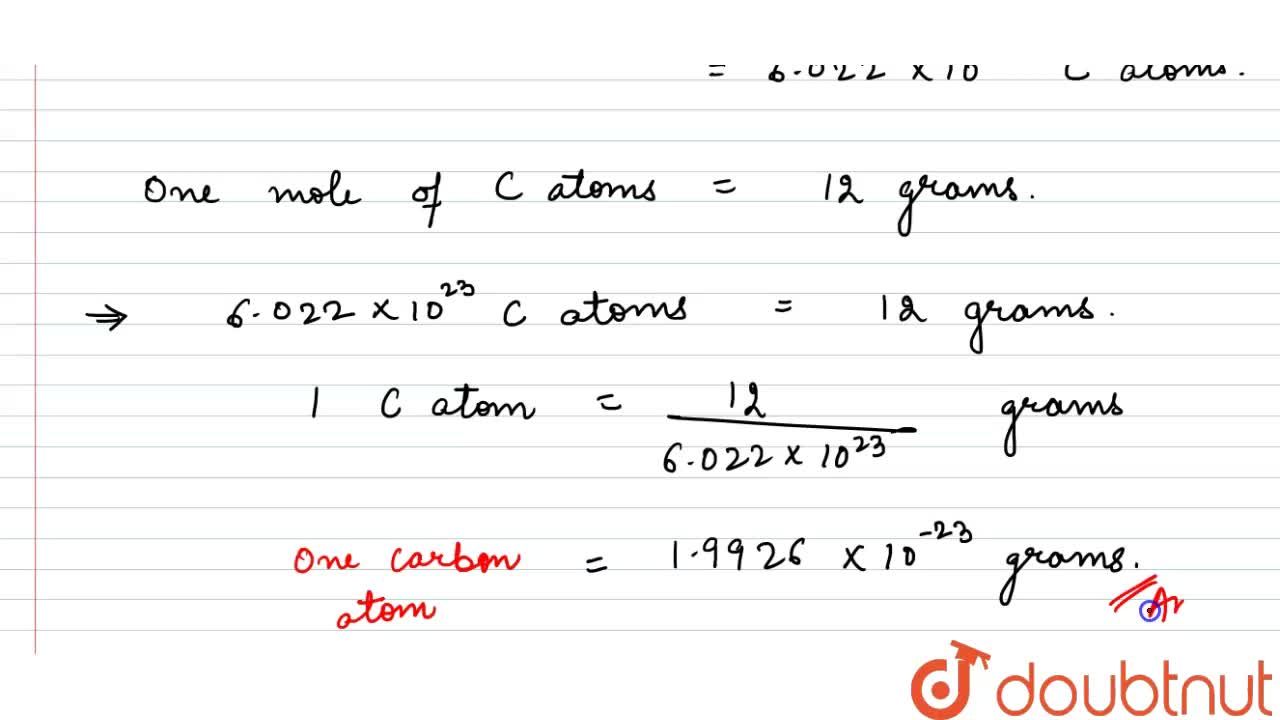 Source: doubtnut.com
Source: doubtnut.com
One mole of carbon-12 atoms has 602214076 10 23 atoms and a mass of 12 grams. This means that the mass of 6022 1023 6022 10 23 atoms of carbon is 12 12 grams. Solution 1 mole of C atoms 6022 10 23 atoms of C 12 g given. A One mole of carbon atoms weigh 12 g. The mass of 1 mole or 12 g.
 Source: toppr.com
Source: toppr.com
12 g 1 atom of carbon-12 That is 1 g 6022 X 1023 amu. Thus the absolute mass of 1 atoms of carbon is 199 1023 199 10 - 23 gram. This means that the mass of 6022 1023 6022 10 23 atoms of carbon is 12 12 grams. 1200 g C-12 1 mol C-12 atoms 6022 1023 atoms The number of particles in 1 mole is called Avogadros Number 60221421 x 1023. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title one mole of carbon weighs 12 grams by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






