Your One mole is equal to one gram images are ready. One mole is equal to one gram are a topic that is being searched for and liked by netizens today. You can Get the One mole is equal to one gram files here. Get all free photos and vectors.
If you’re searching for one mole is equal to one gram images information linked to the one mole is equal to one gram interest, you have pay a visit to the ideal blog. Our site frequently gives you hints for refferencing the highest quality video and image content, please kindly surf and locate more enlightening video articles and graphics that fit your interests.
One Mole Is Equal To One Gram. One may also ask how do I find my mEq weight. The mass of one mole of a substance equals to its relative molecular mass expressed in grams. 6022 140 78 10 23 is also called. It is defined as the number of grams of a substance ie.
 Moles Moles Mole Is A Way Of Translating Between Grams And Amu S A Mole Is A Number That Helps Us Translate Between The Atomic World And Our Ppt Download From slideplayer.com
Moles Moles Mole Is A Way Of Translating Between Grams And Amu S A Mole Is A Number That Helps Us Translate Between The Atomic World And Our Ppt Download From slideplayer.com
2016 grams An amount of any substance in grams that is numerically equal to its atomic or molecular weight in amu has been defined as one mole of that substance. We assume you are converting between moles 1 and gram. The mass of one mole of a substance equals to its relative molecular mass expressed in grams. So this is how many grams we have. 6022 140 78 10 23 is also called. Note that rounding errors may occur so always check the results.
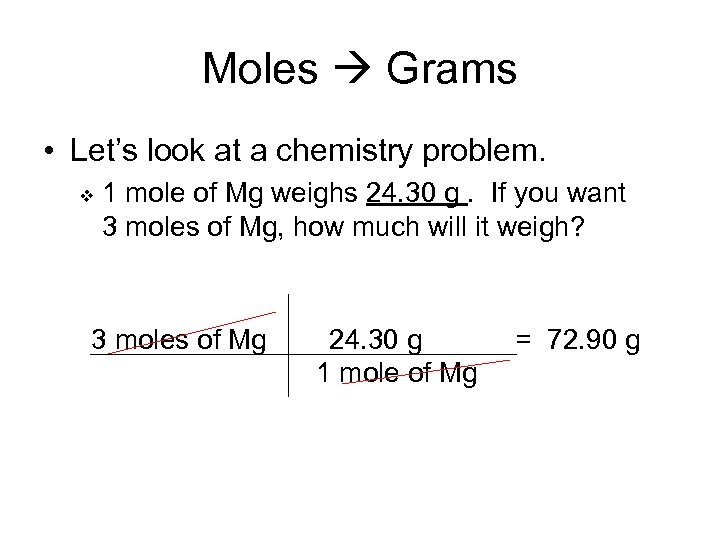
1 mole is equal to 1 moles Mg or 24305 grams.
In one mole there are 6022 times 1023 particles which may be. It is defined as the number of grams of a substance ie. The answer is 247. 1 mole is equal to 1 moles Mg or 24305 grams. Be aware that rounding errors could happen so all the time examine the outcomes. For oxygen which exists in air as the molecule O2 the molecular weight is 16x2 or 32.
 Source: slideplayer.com
Source: slideplayer.com
1 gram is equal to 00227 moles. Molecular weight of 1 or mol The SI base unit for amount of substance is the mole. Every substance has its own unique molegram ratio. 6022 140 78 10 23 is also called. The grams cancel because they are both on the bottom of the equation if you make the amount in grams a fraction over 1 gram which leaves moles and the answer.
 Source: pinterest.com
Source: pinterest.com
The gram-equivalent weight of an electrolyte is the molecular weight of the electrolyte expressed in grams also known as the gram molecular weight or mole divided by the valence of the electrolyte. 15K views View upvotes. And then if we wanna figure out how many moles and its going to be a small fraction of a mole because a mole is 7263 grams per mole we have a small fraction of a gram much less 7263 grams. One mole is around 600 sextillion molecules. The weight of one mole of carbon dioxide is 4401 g.
 Source: present5.com
Source: present5.com
Molecular mass of substance. The grams cancel because they are both on the bottom of the equation if you make the amount in grams a fraction over 1 gram which leaves moles and the answer. How many moles 1 in 1 grams. Note that rounding errors may occur so always check the results. Convert another chemical substance Convert moles to grams.
 Source: slideplayer.com
Source: slideplayer.com
The average mass of one mole of H2O is 1802 grams. Scientists use this number because 1 gram of hydrogen is around 1 mole of atoms. So one gram mole of O2 is 32 grams. It is defined as the number of grams of a substance ie. So one gram mole of CO2 is 44 grams.
 Source: slidetodoc.com
Source: slidetodoc.com
And then if we wanna figure out how many moles and its going to be a small fraction of a mole because a mole is 7263 grams per mole we have a small fraction of a gram much less 7263 grams. So one gram mole of O2 is 32 grams. By this definition one mole of hydrogen is 2016 grams one mole of methane is 16043 grams. 1 mole is equal to 1 moles MgCl2 or 95211 grams. Type in your own numbers in the form to convert the units.
 Source: in.pinterest.com
Source: in.pinterest.com
One may also ask how do I find my mEq weight. A mole is the quantity of a substance where the mass in grams equals the molecular mass so one mole of water weighs 18g. To find the number of particles in 128 moles of potassium simply multiply 128 by Avacado- I mean Avagadros constant. In one mole there are 6022 times 1023 particles which may be. One mole is defined as the amount of substance containing as many elementary entities atoms molecules ions electrons radicals etc as there are atoms in 12 grams of carbon - 126.
 Source: pinterest.com
Source: pinterest.com
One may also ask how do I find my mEq weight. The SI base unit for amount of substance is the mole. Molecular weight of 1 or mol The SI base unit for amount of substance is the mole. You can view more details on each measurement unit. Note that rounding errors may occur so always check the results.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
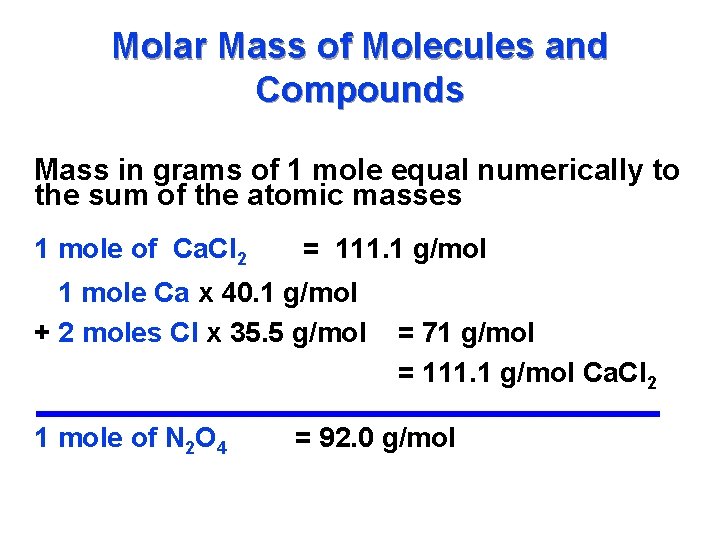
For the molecule CO2 the molecular weight is 121616 or 44. One mEq is equal to 111000 of the gram -equivalent weight GEW of a solute. So one gram mole of CO2 is 44 grams. For oxygen which exists in air as the molecule O2 the molecular weight is 16x2 or 32. Be aware that rounding errors could happen so all the time examine the outcomes.
 Source: slideplayer.com
Source: slideplayer.com
The exact value of one mole is 6022 140 78 10 23This number comes from experiments with carbon because its easy to work with. 1 1 mole of Ammonia 14 3 17g The molecular mass is the same as sum of the atomic lots of all of the atoms current in. So one gram mole of O2 is 32 grams. The SI base unit for amount of substance is the mole. How do you know 1 gram of a substance 1 mole.
 Source: slideplayer.com
Source: slideplayer.com
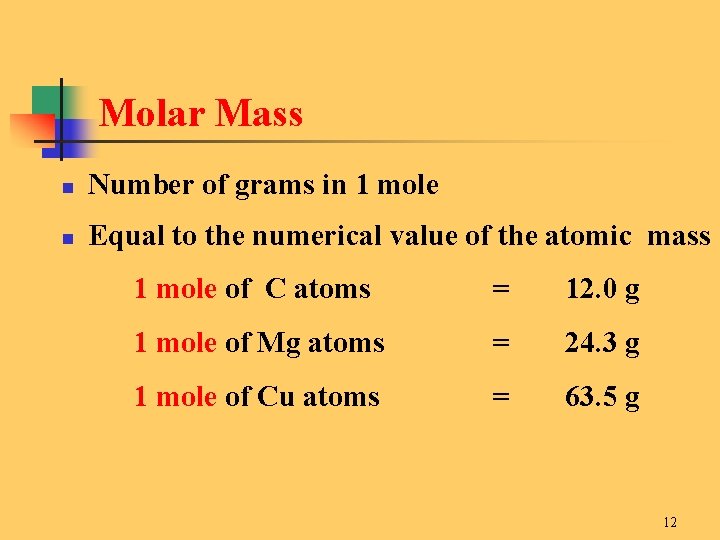
One gram of any molecule is not equal to Avogadros number of molecules. The answer is 247. How many grams 1 in 1 mol. These ratios are the molar masses or atomic masses listed on the periodic table. The mass of one mole of an element equal in grams to the atomic weight.
 Source: slidetodoc.com
Source: slidetodoc.com
Note that rounding errors may occur so always check the results. Every substance has its own unique molegram ratio. One mole is defined as the amount of substance containing as many elementary entities atoms molecules ions electrons radicals etc as there are atoms in 12 grams of carbon - 126. For example as stated in an earlier post Hydrogen is 100794 AMU. So one gram mole of CO2 is 44 grams.
 Source: pinterest.com
Source: pinterest.com
Use this page to learn how to convert between moles Helium and gram. It just so happens that at standard temperature and pressure the density of water is 1g per mL. By this definition one mole of hydrogen is 2016 grams one mole of methane is 16043 grams. Click to see full answer. We assume you are converting between moles 1 and gram.

We assume you are converting between grams 1 and mole. So one gram mole of CO2 is 44 grams. Note that rounding errors may occur so always check the results. One gram of any molecule is not equal to Avogadros number of molecules. What does 1 mole of hydrogen weigh.
 Source: slideplayer.com
Source: slideplayer.com
The grams cancel because they are both on the bottom of the equation if you make the amount in grams a fraction over 1 gram which leaves moles and the answer. For example as stated in an earlier post Hydrogen is 100794 AMU. We assume you are converting between grams 1 and mole. 1 grams 1 is equal to 1 mole. One mole is around 600 sextillion molecules.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
The average mass of one mole of H2O is 1802 grams. The gram-equivalent weight of an electrolyte is the molecular weight of the electrolyte expressed in grams also known as the gram molecular weight or mole divided by the valence of the electrolyte. So this is how many grams we have. One mole is around 600 sextillion molecules. Molecular weight of 1 or mol The SI base unit for amount of substance is the mole.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title one mole is equal to one gram by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






