Your One mole is equal to how many grams images are available. One mole is equal to how many grams are a topic that is being searched for and liked by netizens now. You can Get the One mole is equal to how many grams files here. Find and Download all royalty-free photos and vectors.
If you’re searching for one mole is equal to how many grams images information connected with to the one mole is equal to how many grams topic, you have come to the right site. Our website frequently gives you suggestions for downloading the maximum quality video and picture content, please kindly hunt and locate more enlightening video articles and images that fit your interests.
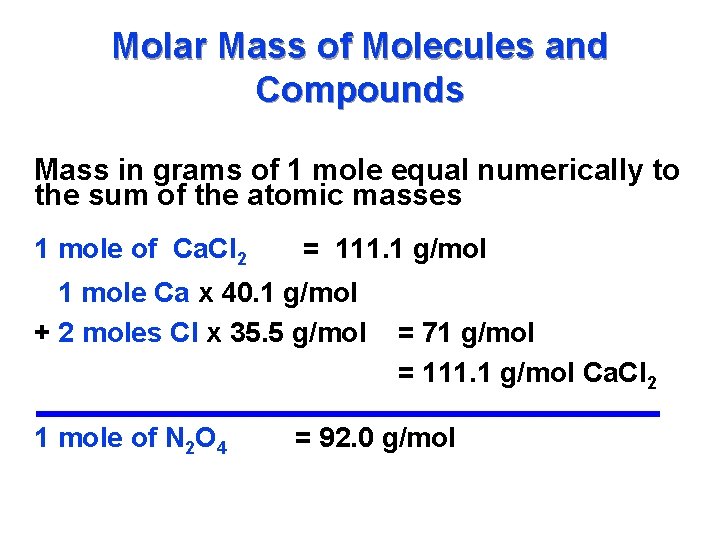
One Mole Is Equal To How Many Grams. And so we saw from our analysis to figure out the number of moles were now going to essentially divide by 7263 so divided by 7263 is equal to this is the number of moles of. One mole of any atom will always have its mass equal to its gram atomic mass. The weight of one mole of carbon dioxide is 4401 g. For example the mean molecular weight of water is 18015 atomic mass units amu so one mole of water weight 18015 grams.

Class 9 molecules are large and complex. For water the molecular weight is 2 of hydrogen and 1 of oxygen 2x11x16 18 so a gram-mole of water weighs 18 grams. Use this page to learn how to convert between grams 1 and mole. Skin problem 0 What type of skin cancer is hereditary. Molecular weight of In or grams The SI base unit for amount of substance is the mole. The weight of one mole of carbon dioxide is 4401 g.
Molecular weight of 1 or mol.
1 mole is equal to 1 moles H2O or 1801528 grams. Molecular weight of In or grams The SI base unit for amount of substance is the mole. 30 moles 1 to grams 30 grams. 100 moles 1 to grams 100 grams. One mole of zinc is. Because one mole of carbon 12 has a mass of 12 grams one mole of carbon is very important in chemistry.
 Source: slideplayer.com
Source: slideplayer.com
A gram-mole is one gram times the molecular weight. And so we saw from our analysis to figure out the number of moles were now going to essentially divide by 7263 so divided by 7263 is equal to this is the number of moles of. Apply the mols mass ar where ar is the atomic relative mass. 30 moles 1 to grams 30 grams. One mole of a material equals one gram of the substances formula mass.
 Source: slideplayer.com
Source: slideplayer.com
40 moles 1 to grams 40 grams. One mole of any atom will always have its mass equal to its gram atomic mass. 1 mole is equal to 1 moles In or 114818 grams. As it is Cl2. 50 moles 1 to grams 50 grams.
 Source: slideplayer.com
Source: slideplayer.com
This 1 mole grams of a substance has a 6023 1023 Avogadros number number of particles. Molecular weight of 1 or mol. Because one mole of carbon 12 has a mass of 12 grams one mole of carbon is very important in chemistry. You can view more details on each measurement unit. The SI base unit for amount of substance is the mole.
 Source: slideplayer.com
Source: slideplayer.com
The molar mass of some substance is the mass in grams of one mole of that substance. 1 grams 1 is equal to 1 mole. One MOLE of hydrogen atoms contains the same number of atoms as the number of hydrogen molecules in one MOLE of hydrogen molecules ie Avagadros number. For example the mean molecular weight of water is 18015 atomic mass units amu so one mole of water weight 18015 grams. And then if we wanna figure out how many moles and its going to be a small fraction of a mole because a mole is 7263 grams per mole we have a small fraction of a gram much less 7263 grams.
 Source: slideplayer.com
Source: slideplayer.com
Molecular weight of 1 or mol. As it is Cl2. Where is the molar mass of the substance. The SI base unit for amount of substance is the mole. Molecular weight of In or grams The SI base unit for amount of substance is the mole.
 Source: slidetodoc.com
Source: slidetodoc.com
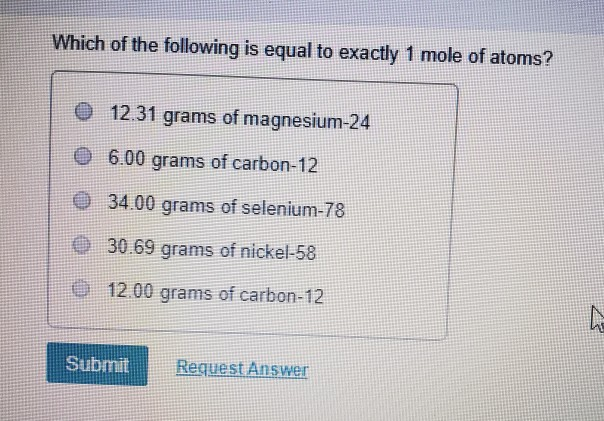
How many is a mole. 1 71 mass. So it tells us that is chlorine gas and the Ar will be 71 instead of 355. One mole of any atom will always have its mass equal to its gram atomic mass. And then if we wanna figure out how many moles and its going to be a small fraction of a mole because a mole is 7263 grams per mole we have a small fraction of a gram much less 7263 grams.

We assume you are converting between moles In and gram. We assume you are converting between moles In and gram. One atom of nitrogen weighs 14 amu. And then if we wanna figure out how many moles and its going to be a small fraction of a mole because a mole is 7263 grams per mole we have a small fraction of a gram much less 7263 grams. They do not affect the number of significant figures.
 Source: slideplayer.com
Source: slideplayer.com
How many moles equal a gram. 100 moles 1 to grams 100 grams. 50 moles 1 to grams 50 grams. Hydrogen atoms have an atomic mass of 100794 so one mole of hydrogen atoms has a mass of 100794 grams. Skin problem 0 What type of skin cancer is hereditary.
 Source: chemistry.wustl.edu
Source: chemistry.wustl.edu
Quick conversion chart of moles 1 to grams. 1 mole is equal to 1 moles Al2O3 or 101961276 grams. 30 moles 1 to grams 30 grams. 1 mole is equal to 1 moles In or 114818 grams. Hydrogen atoms have an atomic mass of 100794 so one mole of hydrogen atoms has a mass of 100794 grams.
 Source: slidetodoc.com
Source: slidetodoc.com
Quick conversion chart of moles 1 to grams. Then convert from moles to grams. 1 gram is equal to 00227 moles. How many moles are in a gram. So it tells us that is chlorine gas and the Ar will be 71 instead of 355.
 Source: objectif-studio.com
Source: objectif-studio.com
We assume you are converting between moles In and gram. However one mole of hydrogen atoms has a mass of 1 gram while one MOLE of hydrogen molecules has a mass of 2 grams. We assume you are converting between moles In and gram. As it is Cl2. In order to convert the moles of a substance to grams you will need to multiply the mole value of the substance by its molar mass.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
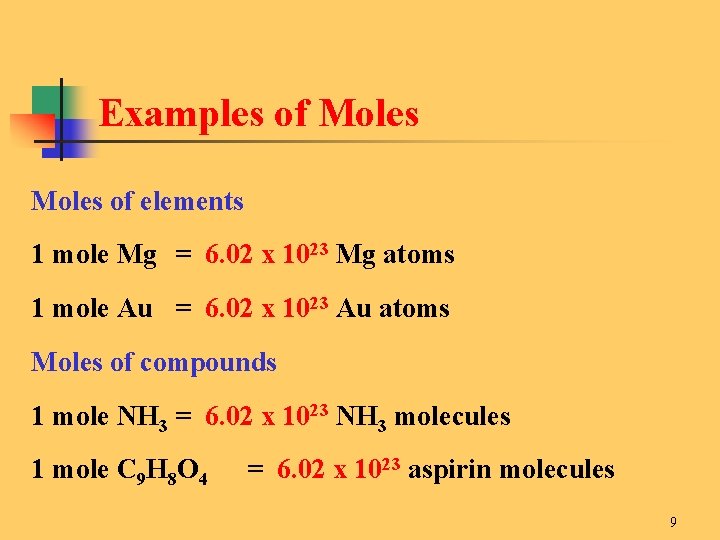
One mole of an element comprises 6022 x 1023 of the elements atoms. For water the molecular weight is 2 of hydrogen and 1 of oxygen 2x11x16 18 so a gram-mole of water weighs 18 grams. 40 moles 1 to grams 40 grams. Where is the molar mass of the substance. Molecular weight of In or grams The SI base unit for amount of substance is the mole.
 Source: slideplayer.com
Source: slideplayer.com
But chemical substances react in stoichiometric ratios of their moles to give any product. We assume you are converting between moles In and gram. 1 mole is equal to 1 moles H2O or 1801528 grams. More information from the unit converter You can view more details on each measurement unit. One mole of a material equals one gram of the substances formula mass.
 Source: chegg.com
Source: chegg.com
Hydrogen atoms have an atomic mass of 100794 so one mole of hydrogen atoms has a mass of 100794 grams. Where is the molar mass of the substance. We assume you are converting between moles In and gram. The SI base unit for amount of substance is the mole. Skin problem 0 What type of skin cancer is hereditary.
 Source: studylib.net
Source: studylib.net
1 mole of nitrogen atoms weigh 14 grams buddy. One mole of a material represents 6022 x 1023 individuals. One mole of a material equals one gram of the substances formula mass. How many is a mole. Where is the molar mass of the substance.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title one mole is equal to how many grams by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.