Your One gram mole of oxygen at 27 images are ready in this website. One gram mole of oxygen at 27 are a topic that is being searched for and liked by netizens now. You can Download the One gram mole of oxygen at 27 files here. Get all free photos.
If you’re searching for one gram mole of oxygen at 27 pictures information linked to the one gram mole of oxygen at 27 topic, you have come to the right site. Our site frequently gives you suggestions for downloading the maximum quality video and image content, please kindly surf and locate more informative video content and images that match your interests.
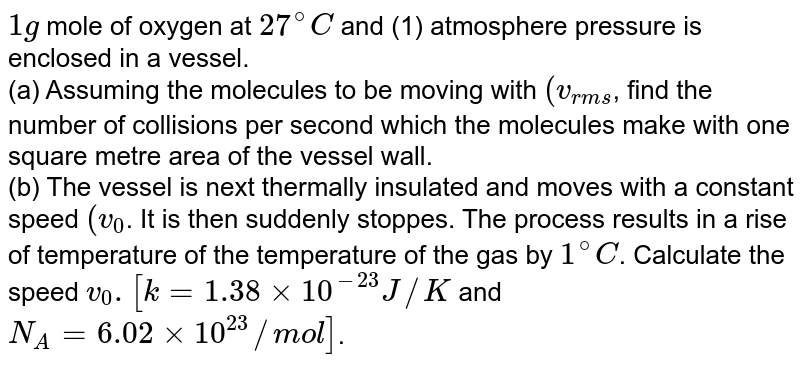
One Gram Mole Of Oxygen At 27. You can also say one mole of sulfuric acid has two mols of hydrogen atoms 1 mol of sulfur atoms and 4 moles of oxygen atoms. Chapter 7 section 3. I Assuming the molecules to be moving the V_ rms Find the number of collisions per second which the molecules make with one square metre area of the vessel wall. A Assuming the molecules to be moving with vfind the number of collisions per second which the mol ecules make with one sqaure metre area of the vessel wal.
 One Gram Mole Of Oxygen At 27 And One Atmospheric Pressure Is Enclosed In Vessel I Assuming The Molecules To Be Moving The V Rms Find The Number Of Collisions Per Second Which From doubtnut.com
One Gram Mole Of Oxygen At 27 And One Atmospheric Pressure Is Enclosed In Vessel I Assuming The Molecules To Be Moving The V Rms Find The Number Of Collisions Per Second Which From doubtnut.com
One gram atom means the mass of one mole of an element equal in grams to the atomic weightOne gram atom of oxygen is defined as the atoms present in one gram of oxygen atom. O 2 is a diatomic molecule. It is then suddenly stopped. One gram mole of oxygen at 27 and one atmospheric pressure is enclosed in vessel. Determine the empirical formula of an oxide of iron which has 699 iron and 301 oxygen by mass. One gram - mole of oxygen at 27 C and 1 atm pressure is enclosed in a vessel.
One sulfuric H2SO4 molecule has 2 hydrogen atoms 1 sulfur atom and 4 oxygen atoms.
In each case multiply the number of moles by Avogadros number 6022 x 10-23 atoms or molecules per mole. But thats in 1 mol of sulfuric acid. One gram-mole of oxygen at 27C and one atmospheric pressure is enclosed in a vessel. Oxygen weighs 0001429 gram per cubic centimeter or 1429 kilogram per cubic meter ie. What is the mass in grams of 100 mole of O2 gas. In Imperial or US customary measurement system the density is equal to 008921 pound per cubic foot lbft³ or 000082601 ounce per cubic inch ozinch³.
 Source: youtube.com
Source: youtube.com
How many moles Oxygen in 1 grams. One gram mole of oxygen at 27C and one atmospheric pressure is enclosed in a vessel. Calculate the average kinetic energy of translation of an oxygen molecule at 27 C the total kinetic energy of an oxygen molecule at 27 C the total kinetic energy in joule of one mole of oxygen at 27 C Given Avogadros number 602 1023 and Boltzmanns constant 138 10-23 J mol - K. A Assuming the molecules to be moving with V rms find the number of collisions per second which molecules make with one square meter area of the vessel wall. The SI base unit for amount of substance is the mole.
 Source: doubtnut.com
Source: doubtnut.com
Ii The vessel is next thermally insulated and moved with a constant speed V 0 V 0. One gram mole of oxygen at 27C and one atmospheric pressure is enclosed in a vessel. 120 colorredcancelcolorblackg overbrace1 mole O_2320colorredcancelcolorblackgcolorpurplemolar mass of molecular oxygen 0375 moles O_2 Your biggest challenge when it comes to this type of problems will often be to make sure that the units given to you match those used in the expression of the. One gram mole of oxygen at 27 and one atmospheric pressure is enclosed in vessel. At 0C 32F or 27315K at standard atmospheric pressure.
 Source: youtube.com
Source: youtube.com
Determine the empirical formula of an oxide of iron which has 699 iron and 301 oxygen by mass. That said to find the mass of one ATOM we need to convert from moles to atoms as follows. The answer is 159994. Molecular weight of Oxygen or grams The molecular formula for Oxygen is O. Mass of one molecule of oxygen.
 Source: toppr.com
Source: toppr.com
This means that 1 MOLE of hydrogen atoms will weigh 1008 grams. One sulfuric H2SO4 molecule has 2 hydrogen atoms 1 sulfur atom and 4 oxygen atoms. One mole equals 602 000 000 000 000 000 000 000 atoms or molecules more concisely written 602 1023. You can view more details on each measurement unit. What is the total mass of oxygen in 100 mole of Al2CrO43.
 Source: youtube.com
Source: youtube.com
I Assuming the molecules to be moving the V rms V r m s Find the number of collisions per second which the molecules make with one square metre area of the vessel wall. One gram mole of oxygen at 27 27 and one atmospheric pressure is enclosed in vessel. 120 colorredcancelcolorblackg overbrace1 mole O_2320colorredcancelcolorblackgcolorpurplemolar mass of molecular oxygen 0375 moles O_2 Your biggest challenge when it comes to this type of problems will often be to make sure that the units given to you match those used in the expression of the. I Assuming the molecules to be moving the V_ rms Find the number of collisions per second which the molecules make with one square metre area of the vessel wall. So in total we have 7 mols of atoms.

Molecular weight of Oxygen or grams The molecular formula for Oxygen is O. The answer is 0062502343837894. One gram mole of oxygen at 27 27 and one atmospheric pressure is enclosed in vessel. I Assuming the molecules to be moving the V_rms Find the number of collisions per second which the molecules make with one square metre area of the vessel wall. What is the mass in grams of 100 mole of O2 gas.
 Source: doubtnut.com
Source: doubtnut.com
We assume you are converting between moles Oxygen and gram. One sulfuric H2SO4 molecule has 2 hydrogen atoms 1 sulfur atom and 4 oxygen atoms. Ii The vessel is next thermally insulated and moved with a constant speed V_0. One gram atom means the mass of one mole of an element equal in grams to the atomic weightOne gram atom of oxygen is defined as the atoms present in one gram of oxygen atom. The SI base unit for amount of substance is the mole.
 Source: doubtnut.com
Source: doubtnut.com
A container is filled with one gm-mole oxygen at a pressure of one at m and temperature 27CIt is assumed that molecules of the gas are moving with velocity VrmsThen Vrmswill be 1 Atoms 105 Nm2 and k 138 x 10-23 JK 148 102 ms 248 102 ms 348 102 cms 448 102 cms. 1 grams Oxygen is equal to 0062502343837894 mole. Assuming the molecules to be moving with vrms the number of collisions per second which the molecules make with 1 m2 area of the vessel wall is approximately N x 1027m-2. O 2 is a diatomic molecule. The SI base unit for amount of substance is the mole.
 Source: doubtnut.com
Source: doubtnut.com
It is then suddenly stopped. 15999 grams the amount of grams in any mole of an element can be found on the periodic table right below the symbol of the element you want it is called the atomic massmolar mass. Calculate the amount of carbon dioxide that can be produced when 1 mole of carbon is burnt in 16 g. One gram mole of oxygen at 27 27 and one atmospheric pressure is enclosed in vessel. The SI base unit for amount of substance is the mole.
 Source: toppr.com
Source: toppr.com
Answer 1 of 3. We assume you are converting between moles Oxygen and gram. What is the total mass of oxygen in 100 mole of Al2CrO43. We assume you are converting between grams Oxygen and mole. The cylinder can be used until its absolute pressure drops to 11 atm.

Mass of O 2 molecule 2 16 32 amu. In the second compound. Ii The vessel is next thermally insulated and moved with a constant speed V 0 V 0. 1008 gramsmole Hydrogen 1 mole6022x1023 atoms 167 x 10-24 grams. Molecular mass is obtained by multiplying the atomic mass of an element with the number of atoms in the molecule and then adding the masses of all the elements in the molecule.

120 colorredcancelcolorblackg overbrace1 mole O_2320colorredcancelcolorblackgcolorpurplemolar mass of molecular oxygen 0375 moles O_2 Your biggest challenge when it comes to this type of problems will often be to make sure that the units given to you match those used in the expression of the. One gram mole of oxygen at 27 and one atmospheric pressure is enclosed in vessel. 1008 gramsmole Hydrogen 1 mole6022x1023 atoms 167 x 10-24 grams. Mass of O 2 molecule 2 16 32 amu. O 2 is a diatomic molecule.
 Source: toppr.com
Source: toppr.com
In Imperial or US customary measurement system the density is equal to 008921 pound per cubic foot lbft³ or 000082601 ounce per cubic inch ozinch³. The cylinder can be used until its absolute pressure drops to 11 atm. Calculate the average kinetic energy of translation of an oxygen molecule at 27 C the total kinetic energy of an oxygen molecule at 27 C the total kinetic energy in joule of one mole of oxygen at 27 C Given Avogadros number 602 1023 and Boltzmanns constant 138 10-23 J mol - K. That said to find the mass of one ATOM we need to convert from moles to atoms as follows. One sulfuric H2SO4 molecule has 2 hydrogen atoms 1 sulfur atom and 4 oxygen atoms.

One mole equals 602 000 000 000 000 000 000 000 atoms or molecules more concisely written 602 1023. It is then suddenly stopped. So the ratio between the masses of C and O 273. 1008 gramsmole Hydrogen 1 mole6022x1023 atoms 167 x 10-24 grams. What is the total mass of oxygen in 100 mole of Al2CrO43.
 Source: doubtnut.com
Source: doubtnut.com
15999 grams the amount of grams in any mole of an element can be found on the periodic table right below the symbol of the element you want it is called the atomic massmolar mass. So in total we have 7 mols of atoms. One gram-mole of oxygen at 27C and one atmospheric pressure is enclosed in a vessel. What is the total mass of oxygen in 100 mole of Al2CrO43. I Assuming the molecules to be moving the V rms V r m s Find the number of collisions per second which the molecules make with one square metre area of the vessel wall.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title one gram mole of oxygen at 27 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






