Your Moles to grams calculator h20 images are available. Moles to grams calculator h20 are a topic that is being searched for and liked by netizens now. You can Download the Moles to grams calculator h20 files here. Get all royalty-free vectors.
If you’re looking for moles to grams calculator h20 pictures information related to the moles to grams calculator h20 keyword, you have come to the ideal site. Our website always provides you with suggestions for seeing the highest quality video and picture content, please kindly surf and find more informative video articles and images that match your interests.
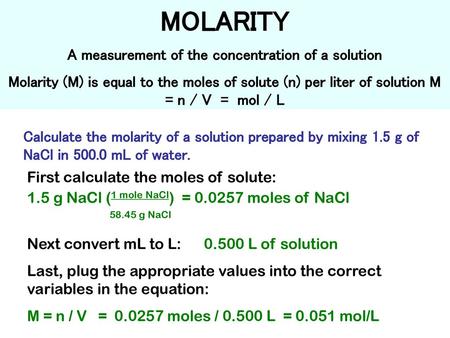
Moles To Grams Calculator H20. Molar Mass of 5 Litres of water 5 18015. Also the Avogadro constant is 602 x 10 23. 2 moles H20 to grams 403176 grams. As you already know how the grams to moles conversion work find the number of moles.
 Grams To Moles Calculator From omnicalculator.com
Grams To Moles Calculator From omnicalculator.com
You may wish to pause and calculate this value if you desire the practice. 25 grams x 1 mol 1341 grams 00093 mol. The mass in grams of a single mole of any compound will equal the molecular weight of the compound when expressed in AMUs. I cant figure it out. 7 moles H20 to grams 1411116 grams. Therefore 1 mole of H2O got 18 grams.
M 3899 107868.
I know the answer is 495moles. You want to calculate how many moles of H2 you have. Track your food intake exercise sleep and meditation for free. I cant figure it out. Molar mass of H20 is 201588 gmol. N 5988 g 18015 gmol 3324 mol.
 Source: slideplayer.com
Source: slideplayer.com
It uses molar volume of a gas at STP standard temperature and pressure ABV Alcohol by volume This online calculator estimates alcohol by volume abv based on original and final specific gravity values. Thus 1 kg of water or ice has 5555 moles of H20. If you have 125 grams of molecules with a molecular weight of 1341 gmol how many moles do you have. 6 moles H20 to grams 1209528 grams. I cant figure it out.
 Source: youtube.com
Source: youtube.com
Do a quick conversion. We count in moles. M 3899 107868. Do a quick conversion. This feature simplifies many stoichiometric calculations.
 Source: toppr.com
Source: toppr.com
5 moles H20 to grams 100794 grams. How many moles equal a gram. The mass in grams of a single mole of any compound will equal the molecular weight of the compound when expressed in AMUs. Thus 1 kg of water or ice has 5555 moles of H20. For example the water molecular weight is 18015 atomic mass units AMU so a mole of water weighs 18015 grams.
 Source: slideplayer.com
Source: slideplayer.com
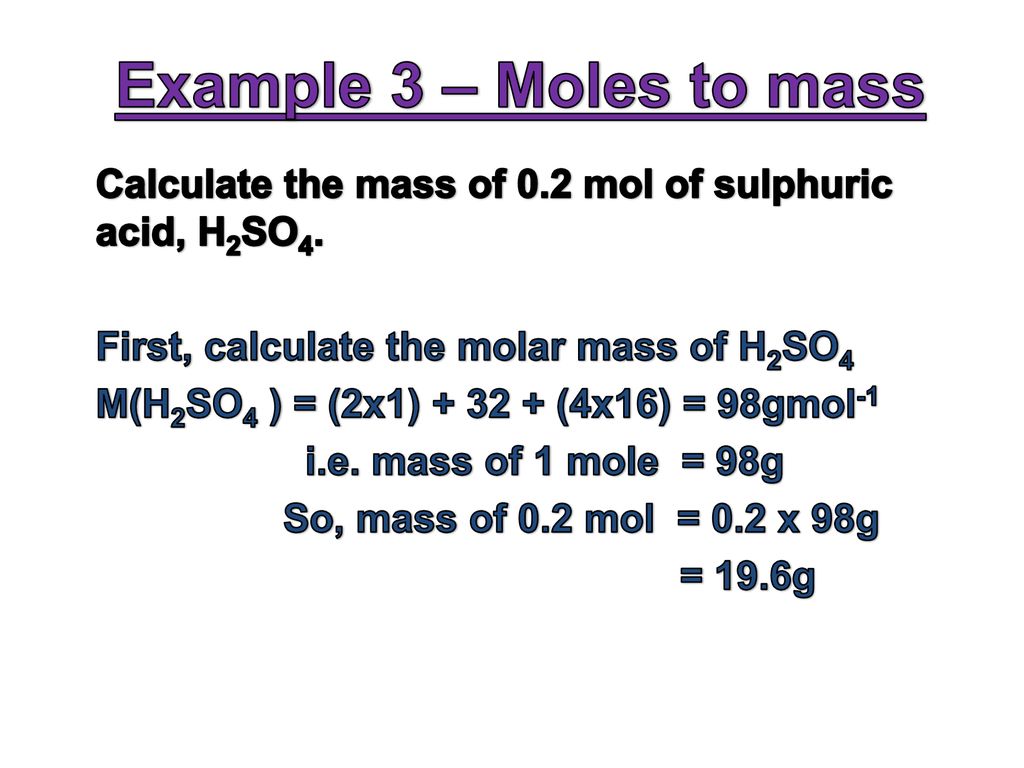
2H2 O2 2H20 answer the following. If you have 125 grams of molecules with a molecular weight of 1341 gmol how many moles do you have. This comes out be 5555 moles. The SI unit for amount of substance is the mole. 02 2 H2 2 H20 1 mol of oxygen is needed to react with 2 moles of Hydrogen to form 2 moles of Water.
 Source: slideplayer.com
Source: slideplayer.com
How many moles of electrons are in 1 kg. 31 Related Question Answers Found. You may wish to pause and calculate this value if you desire the practice. For calculations tap Molar Mass Calculator By using moles to grams formula. 5988 kg 5988 g.
 Source: docsity.com
Source: docsity.com
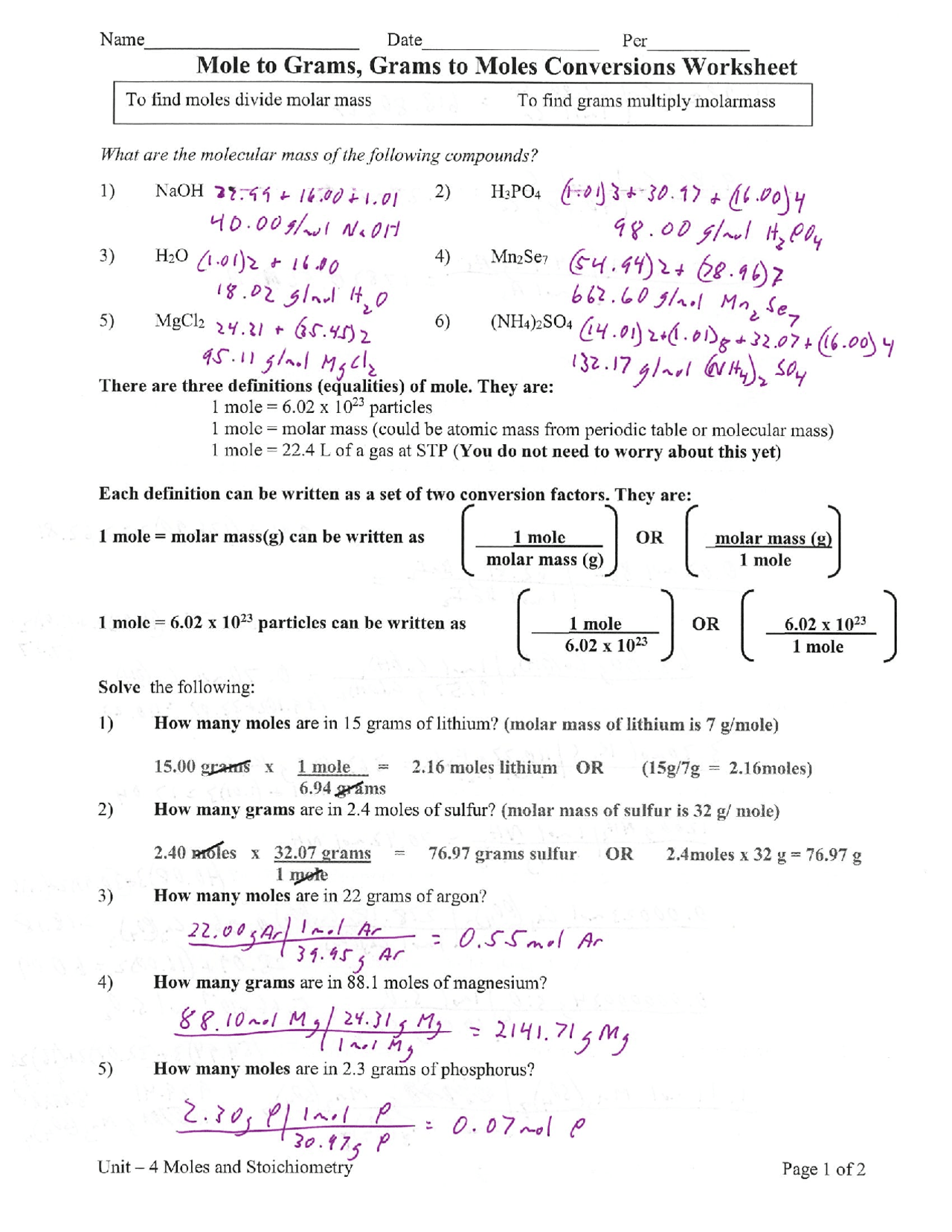
0700 mole x 340146 gramsmole 238 grams. So the conversion of carbon from grams to atoms is given by 10112 602 x 10 23 502 x 10 23. 02 2 H2 2 H20 1 mol of oxygen is needed to react with 2 moles of Hydrogen to form 2 moles of Water. 25 grams x 1 mol 1341 grams 00093 mol. This feature simplifies many stoichiometric calculations.
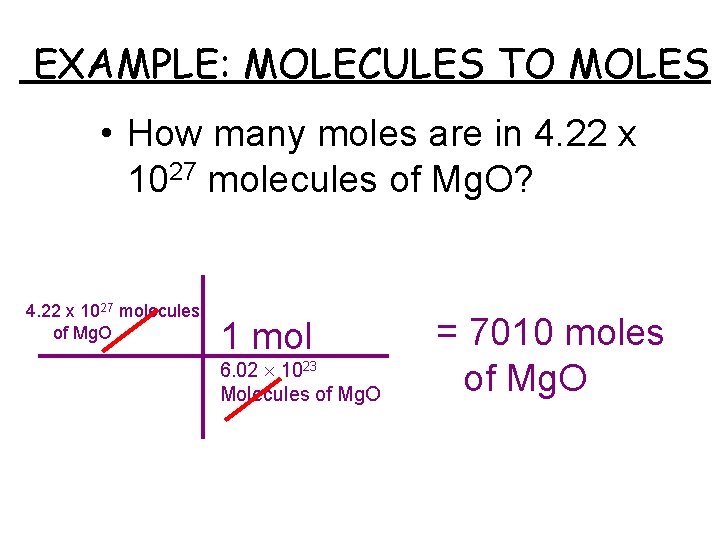 Source: slidetodoc.com
Source: slidetodoc.com
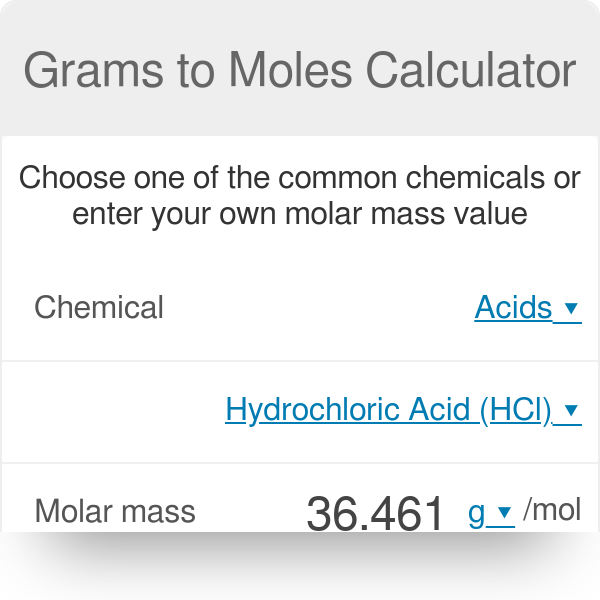
It is a unit factor that we use for converting from grams to moles and moles to grams. 25 grams x 1 mol 1341 grams 00093 mol. Convert between H20 weight and moles. Suppose you have 6 moles of H20. We count in moles.
 Source: youtube.com
Source: youtube.com
Therefore 1 mole of H2O got 18 grams. 1 mole is equal to 1 moles In or 114818 grams. M 3899 107868. It uses molar volume of a gas at STP standard temperature and pressure ABV Alcohol by volume This online calculator estimates alcohol by volume abv based on original and final specific gravity values. It means that 12 grams per mole.
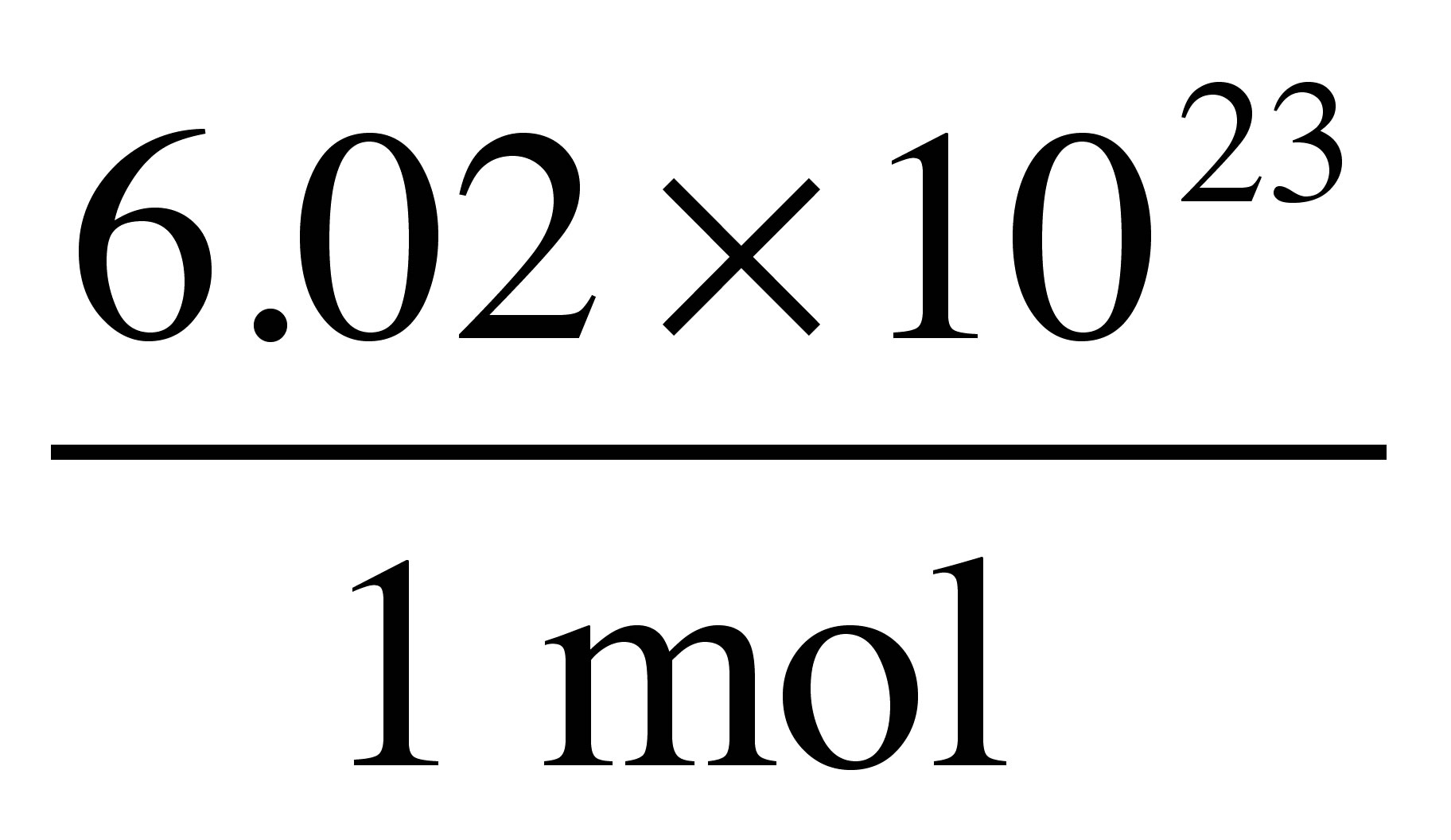 Source: socratic.org
Source: socratic.org
How many is it. We know that the molar mass of 1 mole of carbon is equal to 12. For calculations tap Molar Mass Calculator By using moles to grams formula. 5988 kg 5988 g. This comes out be 5555 moles.
 Source: slideplayer.com
Source: slideplayer.com
Therefore 1 mole of H2O got 18 grams. Suppose you have 6 moles of H20. One mole of any substance will have 60221023 molecules of that substance. Remember that the Avogadro constant is defined as the number of constituent particles atom or formula unit per mole of a given substance. Convert from moles to grams 2172 mol AgBr 1878 g AgBr 1 mol AgBr 4079 g AgBr This is the theoretical yield Yields 22 bCalculate the percent yield if 3750 g of silver bromide was obtained from the reaction theoretical yield 4079 g AgBr percent yield 100 x actual yield theoretical yield percent yield 100 x 3750 g.
 Source: khanacademy.org
Source: khanacademy.org
Track your food intake exercise sleep and meditation for free. How many moles of electrons are in 1 kg. M n M. 1 mole is equal to 1 moles In or 114818 grams. 1 mole of hydrogen atoms got 1 gram so 2 moles of them got 2 grams.
 Source: youtube.com
Source: youtube.com
Thus 1 kg of water or ice has 5555 moles of H20. M 6 l 998 kgm³ 0006 m³ 998 kgm³ 5988 kg. For calculations tap Molar Mass Calculator By using moles to grams formula. 5 moles H20 to grams 100794 grams. Remember that the Avogadro constant is defined as the number of constituent particles atom or formula unit per mole of a given substance.
 Source: omnicalculator.com
Source: omnicalculator.com
Track your food intake exercise sleep and meditation for free. Molecular weight of In or grams The SI base unit for amount of substance is the mole. I cant figure it out. Calculation process for converting grams to moles using conversion factors for stoichiometry. You can view more details on each measurement unit.
 Source: youtube.com
Source: youtube.com
Moles from grams 2 Answers Given this equation. 5 moles H20 to grams 100794 grams. This online calculator converts grams to liters and liters to grams given a gas formula. The technique used can be applied to any mole to gram conversion. Therefore 1 mole of H2O got 18 grams.
 Source: youtube.com
Source: youtube.com
1 mole is equal to 1 moles In or 114818 grams. 5988 kg 5988 g. 02 2 H2 2 H20 1 mol of oxygen is needed to react with 2 moles of Hydrogen to form 2 moles of Water. Convert between H20 weight and moles. Molar Mass of 1 Litre of water 18015.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title moles to grams calculator h20 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.