Your Moles to grams calculator carbon images are ready in this website. Moles to grams calculator carbon are a topic that is being searched for and liked by netizens today. You can Download the Moles to grams calculator carbon files here. Find and Download all free vectors.
If you’re looking for moles to grams calculator carbon images information linked to the moles to grams calculator carbon interest, you have pay a visit to the ideal site. Our site frequently provides you with hints for seeking the maximum quality video and picture content, please kindly surf and find more enlightening video content and graphics that fit your interests.
Moles To Grams Calculator Carbon. Molar mass of NaCl is 58443 how many grams is 5 mole NaCl. Suppose you have 10 gram of carbon. This converts atomic units to grams per mole making the molar mass of hydrogen 1007 grams per mole of carbon 120107 grams. 5 moles Carbon Dioxide to grams 2200475 grams.
 Calculation Of Number Of Moles And Number Of Molecules And Atoms From thefactfactor.com
Calculation Of Number Of Moles And Number Of Molecules And Atoms From thefactfactor.com
4 moles Carbon Dioxide to grams 176038 grams. Moles to molecules calculator scientific notation. Molar mass of NaCl is 58443 how many grams is 5 mole NaCl. Determine the moles of carbon. The amount of moles in a substance can be determined using that substances molar mass. Amazingly there are 602x1023 atoms in each of the samples aboveA sample of 12 grams of carbon is equal to one mole.
Density of carbon dioxide is equal to 1836 kgm³.
Determine the moles of carbon. Meyo - meet you chat game live. Moles to molecules calculator scientific notation. Carbon dioxide weighs 0001836 gram per cubic centimeter or 1836 kilogram per cubic meter ie. We know that the molar mass of 1 mole of carbon is equal to 12. Also the Avogadro constant is 602 x 1023.
 Source: inchcalculator.com
Source: inchcalculator.com
1200 g C-12 1 mol C-12 atoms 6022 1023 atoms The number of particles in 1 mole is called Avogadros Number 60221421 x 1023. We know that the molar mass of 1 mole of carbon is equal to 12. The mass in grams of a compound is equal to its molarity in moles multiply its molar mass. Then multiply the moles by Avogadros number of atoms. The molar mass is the amount of grams in one mole of a substance.
 Source: uctsc.org
Source: uctsc.org
6 moles Carbon Dioxide to grams 264057 grams. Quick conversion chart of moles Carbon Dioxide to grams. Using a calculator divide the number of grams by the molar mass. As you already know how the grams to moles conversion work find the number of moles. Substances react in simple ratios of moles.
 Source: uctsc.org
Source: uctsc.org
Now we have to perform moles to grams calculation. Density of carbon dioxide is equal to 1836 kgm³. Using a calculator divide the number of grams by the molar mass. 4 moles Carbon to grams 480428 grams. 2 moles Carbon to grams 240214 grams.
 Source: youtube.com
Source: youtube.com
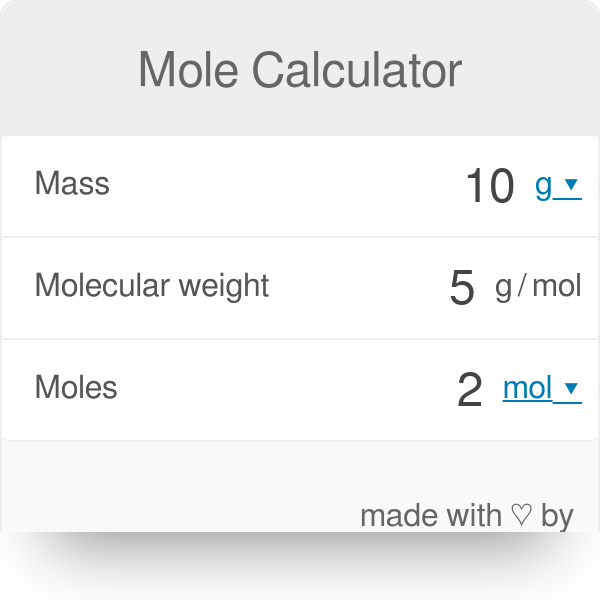
For example imagine you have 2 g of water or H 2 O and you want to convert it to moles. Substances react in simple ratios of moles. Calculate the volume of carbon dioxide gas CO 2 occupied by a 5 moles and b 05 moles of the gas occupied at STP. 1 mole of carbon multiplied by 12011 means you have 12011 grams of carbon atoms. A mole of a substance or a mole of particles is defined as exactly 60221407610²³ particles which may be atoms molecules ions or electrons.
 Source: omnicalculator.com
Source: omnicalculator.com
8 moles ch3cooh to grams 48041568 grams. Quick conversion chart of moles Carbon to grams. 3 moles Carbon Dioxide to grams 1320285 grams. Home Uncategorized moles to molecules calculator scientific notation. It means that 12 grams per mole.
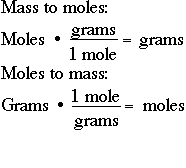 Source: uctsc.org
Source: uctsc.org
6 moles Carbon Dioxide to grams 264057 grams. 12g CO 2 X 2 atoms O 055mols 44 gmol 1 molecule CO 2. 6 moles Carbon to grams 720642 grams. Then multiply the moles by Avogadros number of atoms. 5844 g of Sodium Chloride NaCl 1 Mole of Sodium Chloride NaCl 602214076 8 1023 number of Formula Units.
 Source: uctsc.org
Source: uctsc.org
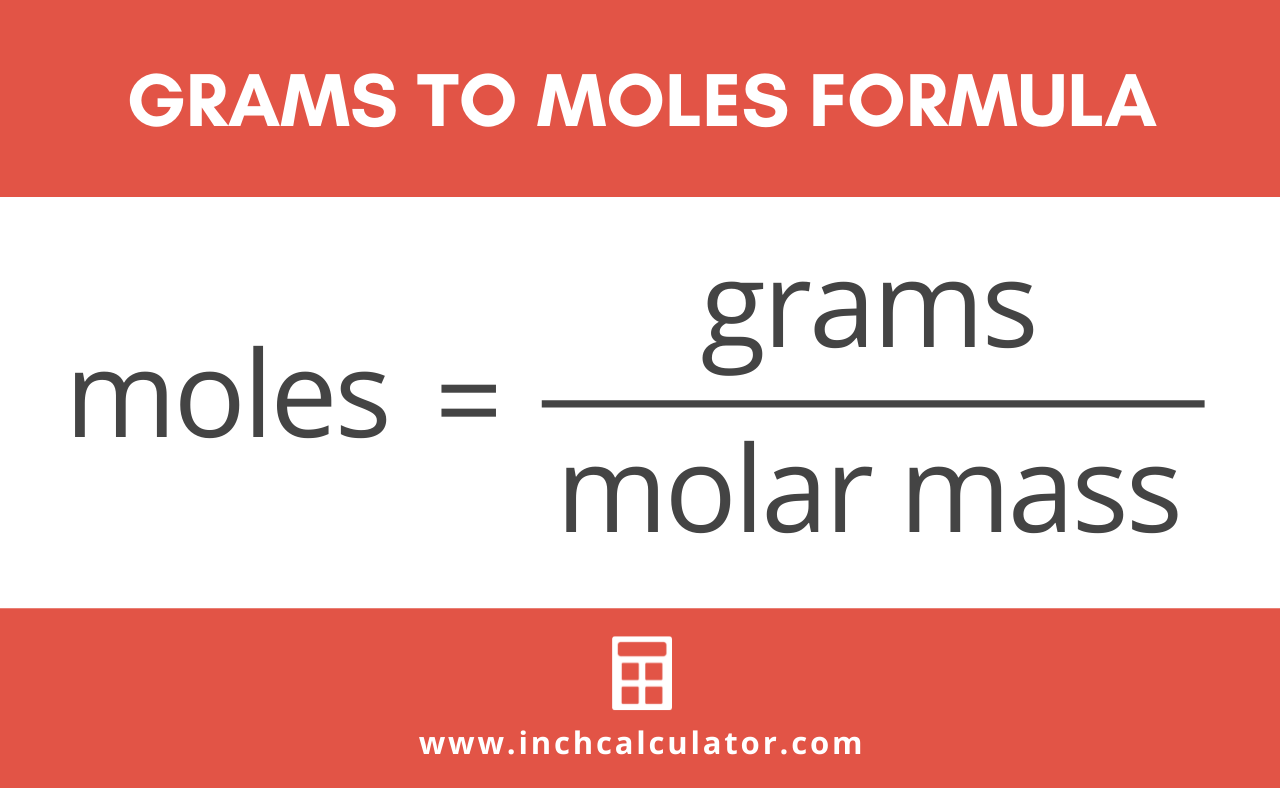
At 25C 77F or 29815K at standard atmospheric pressure. How Many Molecules in a Mole. Grams mole molar mass. 1 mole of carbon multiplied by 12011 means you have 12011 grams of carbon atoms. Home Uncategorized moles to molecules calculator scientific notation.
 Source: youtube.com
Source: youtube.com
5 moles Carbon to grams 600535 grams. The molecular mass of H 2 O is 18gmol. The molar mass is the amount of grams in one mole of a substance. At 25C 77F or 29815K at standard atmospheric pressure. Grams mole molar mass.
 Source: uctsc.org
Source: uctsc.org
Density of carbon dioxide is equal to 1836 kgm³. The molar mass is the amount of grams in one mole of a substance. 6 moles Carbon to grams 720642 grams. 5844 g of Sodium Chloride NaCl 1 Mole of Sodium Chloride NaCl 602214076 8 1023 number of Formula Units. 2 moles Carbon to grams 240214 grams.
 Source: uctsc.org
Source: uctsc.org
10 112 602 x 1023 502 x 1023. M n M m 3899 107868 m 420577332 g You can also verify the results by fetching the values in our free moles to grams calculator. 3 moles Carbon to grams 360321 grams. 07082017 Apply your answer from Steps 2 and 3. Multiply the number of moles by the atomic or molecular mass moles are alway gram moles unless otherwise specified.
 Source: uctsc.org
Source: uctsc.org
Moles to molecules calculator scientific notation. Suppose you have 10 gram of carbon. Grams mole molar mass. Grams to atoms to moles. How to Calculate the Stoichiometric Ratio Sciencing.
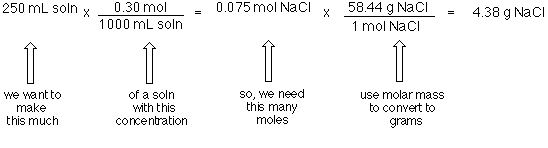 Source: uctsc.org
Source: uctsc.org
12g CO 2 X 2 atoms O 055mols 44 gmol 1 molecule CO 2. 3 moles Carbon to grams 360321 grams. Substances react in simple ratios of moles. Calculate moles of carbon in 100 g. The value of the mole is equal to the number of atoms in exactly 12 grams of pure carbon-12.
 Source: thefactfactor.com
Source: thefactfactor.com
3 moles Carbon Dioxide to grams 1320285 grams. Using a calculator divide the number of grams by the molar mass. This converts atomic units to grams per mole making the molar mass of hydrogen 1007 grams per mole of carbon 120107 grams. For example imagine you have 2 g of water or H 2 O and you want to convert it to moles. Meyo - meet you chat game live.
 Source: surfguppy.com
Source: surfguppy.com
Calculate the volume of carbon dioxide gas CO 2 occupied by a 5 moles and b 05 moles of the gas occupied at STP. Then multiply the moles by Avogadros number of atoms. O 16 mg 24 solution. Grams to atoms to moles. Now we have to perform moles to grams calculation.
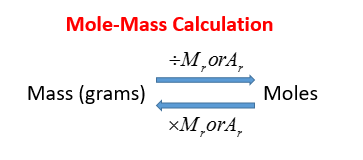 Source: onlinemathlearning.com
Source: onlinemathlearning.com
1 10 -6. Mole is the standard unit of amount measurement. 5844 g of Sodium Chloride NaCl 1 Mole of Sodium Chloride NaCl 602214076 8 1023 number of Formula Units. Grams mole molar mass. A mole of a substance or a mole of particles is defined as exactly 60221407610²³ particles which may be atoms molecules ions or electrons.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title moles to grams calculator carbon by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






