Your Molecular weight is in grams or grams per mole images are ready in this website. Molecular weight is in grams or grams per mole are a topic that is being searched for and liked by netizens now. You can Download the Molecular weight is in grams or grams per mole files here. Get all free photos.
If you’re looking for molecular weight is in grams or grams per mole pictures information connected with to the molecular weight is in grams or grams per mole topic, you have visit the right site. Our site frequently provides you with hints for refferencing the highest quality video and image content, please kindly search and locate more informative video articles and graphics that match your interests.
Molecular Weight Is In Grams Or Grams Per Mole. To determine the number of grams we need we multiply the number of moles by the molecular weight which is grams per mole. A mass in grams equal to a substances molecular weight or the sum of all the atomic weights in its molecular formula The mass in grams of one mole of atoms in a monatomic chemical element is known as gram atomic mass. Example 1 Calculate the mass in grams of 36 mol of H 2 SO 4. So we can multiply the five moles needed by 75 grams per mole and we can solve that we need 375 grams of the substance.
 Density To Molarity Conversion Chemistry Density Conversation From pinterest.com
Density To Molarity Conversion Chemistry Density Conversation From pinterest.com
The unit of molar mass is gramsmole. Furthermore if you dissolve 1 mole of a substance in enough water to make 1 literL of solution you have made a 1-molar1 M solution. Since the unified atomic mass unit symbol. The formula for moles to grams is given by. So we can multiply the five moles needed by 75 grams per mole and we can solve that we need 375 grams of the substance. Its submitted by handing out in the best field.
1 gram is then equal to 292 millimoles of sucrose.
Calculate the mass of 4 moles of aluminium. Another property of Avogadros number is that the mass of one mole of a substance is equal to that substances molecular weight. And we could say grams of glucose C6H12O6 per mole of glucose C6H12O6 and then we can use this 152 kilograms to figure out how many moles we have. Calculate the mass of 4 moles of aluminium. The molecular mass of sucrose C12H22O11 is 342296 grams per mole. We identified it from well-behaved source.
 Source: bqua.com
Source: bqua.com
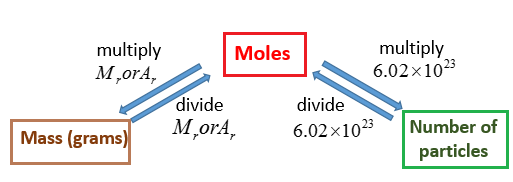
We receive this kind of Molecular Weight To Moles graphic could possibly be the most trending topic taking into account we part it in google improvement or facebook. We receive this kind of Molecular Weight To Moles graphic could possibly be the most trending topic taking into account we part it in google improvement or facebook. The formula for moles to grams is given by. And we could say grams of glucose C6H12O6 per mole of glucose C6H12O6 and then we can use this 152 kilograms to figure out how many moles we have. Kilogram per mole kgmol 10 -3.
 Source: pinterest.com
Source: pinterest.com
The molecular mass of sucrose C12H22O11 is 342296 grams per mole. In calculations this allows us to go back and forth between the amount of substance expressed in grams and the amount of substance expressed in number of molecules as it is when youre dealing with moles. If you know the quantity of mole it can be converted into grams and vice versa. Carbon is out there within the atomic type. Kilogram per mole kgmol 10 -3.

So we can multiply the five moles needed by 75 grams per mole and we can solve that we need 375 grams of the substance. We receive this kind of Molecular Weight To Moles graphic could possibly be the most trending topic taking into account we part it in google improvement or facebook. We can use this to get from a quantity expressed in grams like 74 grams of water a macroscopic quantity that we might weigh out in a lab to the quantity expressed in. Look for the atomic masses of hydrogen sulfur and oxygen. We identified it from well-behaved source.
 Source: pinterest.com
Source: pinterest.com
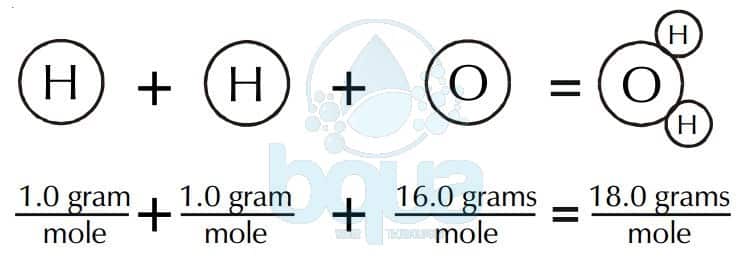
The gram molecular mass of water is 18 grams per mole. In calculations this allows us to go back and forth between the amount of substance expressed in grams and the amount of substance expressed in number of molecules as it is when youre dealing with moles. Mass g No. Therefore the molecular mass of H 2 SO 4 is. The unit of molar mass is gramsmole.
 Source: br.pinterest.com
Source: br.pinterest.com
How do you convert molarity to grams per mole. Mass g No. The units used are grams per mole because the molecular weight is usually expressed as. Gram per mole gmol 1. We identified it from well-behaved source.
 Source: bqua.com
Source: bqua.com
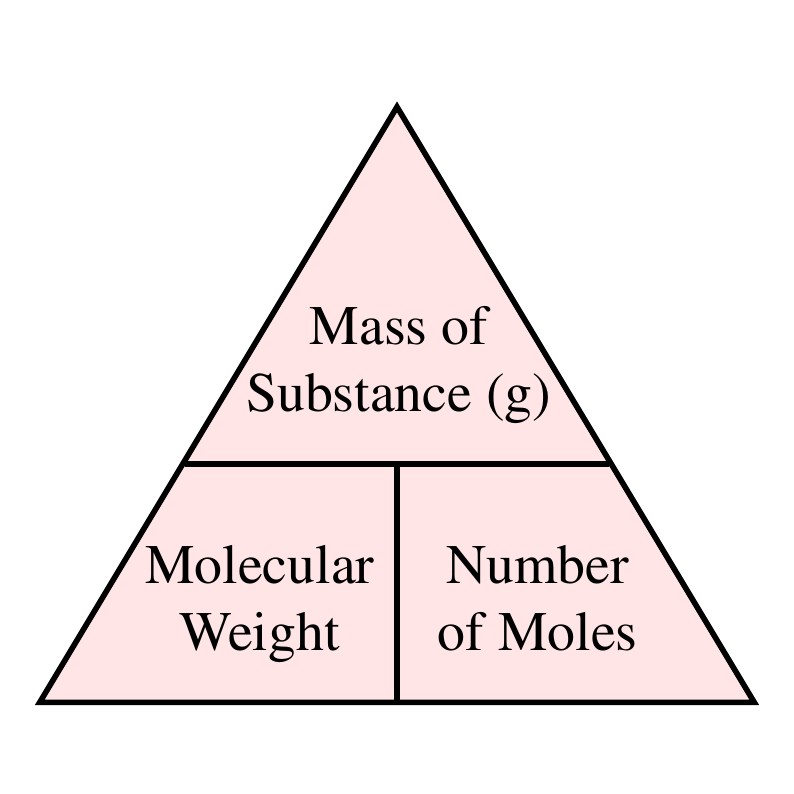
For example the mean molecular weight of water is 18015 atomic mass units amu so one mole of water weight 18015 grams. The molar massmolecular weight is actually the sum of the total mass in grams of the atoms present to make up a molecule per mole. Example 1 Calculate the mass in grams of 36 mol of H 2 SO 4. Moles mol x Molar Mass gmol 1 x 5844. This gives us the two ratios shown in the image below which we can use to convert between grams and moles.
 Source: ncl.ac.uk
Source: ncl.ac.uk
The unit of molar mass is gramsmole. A substance is something that has mass and occupies space. Another property of Avogadros number is that the mass of one mole of a substance is equal to that substances molecular weight. Since the unified atomic mass unit symbol. This isbecause a water molecule contains two hydrogen atoms one protoneach and one oxygen atom 8 protons and 8 neutrons for a totalof 18 nucleons.
 Source: pinterest.com
Source: pinterest.com
I can only go to the hundredths place for significant figures so 18016. The units used are grams per mole because the molecular weight is usually expressed as. Formula to convert moles to grams. Another property of Avogadros number is that the mass of one mole of a substance is equal to that substances molecular weight. So thats equal to 18016 grams per mole.
 Source: study.com
Source: study.com
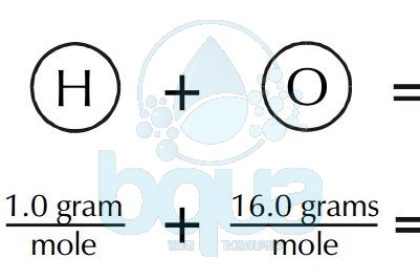
A substance is something that has mass and occupies space. We can use this to get from a quantity expressed in grams like 74 grams of water a macroscopic quantity that we might weigh out in a lab to the quantity expressed in. The molecular weight is the number of grams per mole of a substance or less formally how much a gram of the substance would weigh on the earths surface. Carbon is out there within the atomic type. This property simplifies many chemical computations.
 Source: pinterest.com
Source: pinterest.com
A mole is the quantity of a substance whose weight in grams is equal to the molecular weight of the substance. First you must calculate the number of moles in this solution by rearranging the equation. Moles mol Molarity M x Volume L 05 x 2. For example the mean molecular weight of water is 18015 atomic mass units amu so one mole of water weight 18015 grams. The mass of a grams of a molecule.
 Source: pinterest.com
Source: pinterest.com
Avogadros number is the number of water moleculesneeded to obtain a mass of 18 grams. Another property of Avogadros number is that the mass of one mole of a substance is equal to that substances molecular weight. So we can multiply the five moles needed by 75 grams per mole and we can solve that we need 375 grams of the substance. First you must calculate the number of moles in this solution by rearranging the equation. Avogadros number is the number of water moleculesneeded to obtain a mass of 18 grams.
 Source: pinterest.com
Source: pinterest.com
The molecular mass of sucrose C12H22O11 is 342296 grams per mole. This property simplifies many chemical computations. Therefore the molecular mass of H 2 SO 4 is. We know that the molecular weight of our substance is 75 grams per mole and that we need 5 moles. For NaCl the molar mass is 5844 gmol.
 Source: pinterest.com
Source: pinterest.com
Molecular Weight To Moles - 9 images - molar mass molecular weight of n2o youtube how to convert grams to moles video lesson transcript. Mole Ratio Straightforward Science tenth Grade Science Mole Straightforward Science The molecular weight of water is eighteen amu so one mole of water has a mass of 18 grams. 5 rows We calculate the molar mass of one water molecule when we add 2 x 1 16 18 grams. First you must calculate the number of moles in this solution by rearranging the equation. For NaCl the molar mass is 5844 gmol.
 Source: onlinemathlearning.com
Source: onlinemathlearning.com
At STP within the items grams per liter. At STP within the items grams per liter. To determine the number of grams we need we multiply the number of moles by the molecular weight which is grams per mole. First you must calculate the number of moles in this solution by rearranging the equation. Therefore the molecular mass of H 2 SO 4 is.
 Source: pinterest.com
Source: pinterest.com
Look for the atomic masses of hydrogen sulfur and oxygen. In calculations this allows us to go back and forth between the amount of substance expressed in grams and the amount of substance expressed in number of molecules as it is when youre dealing with moles. How do you convert molarity to grams per mole. This property simplifies many chemical computations. Molecular weight of 15756 grams per mole and also you get seven level eight eight grams.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title molecular weight is in grams or grams per mole by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






