Your Molecular weight in grams per mole images are ready. Molecular weight in grams per mole are a topic that is being searched for and liked by netizens now. You can Find and Download the Molecular weight in grams per mole files here. Find and Download all free images.
If you’re searching for molecular weight in grams per mole pictures information related to the molecular weight in grams per mole interest, you have pay a visit to the right blog. Our website frequently provides you with hints for refferencing the highest quality video and picture content, please kindly surf and find more enlightening video content and images that match your interests.
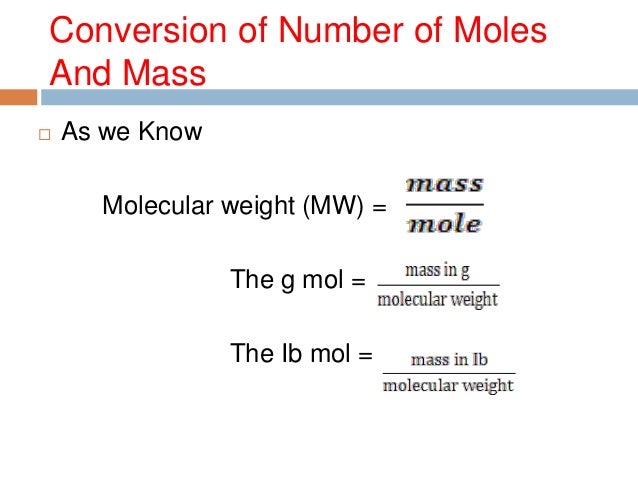
Molecular Weight In Grams Per Mole. The density of one mole in grams is the weight in atomic mass units of that element. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. Grams per mole to Kilograms per mole gmol to kgmol converter.
 Mole From slideshare.net
Mole From slideshare.net
Oxygen we can see from our periodic table of elements it has a molar mass of 1600 grams per mole. Grams per mole to Kilograms per mole gmol to kgmol converter. This isbecause a water molecule contains two hydrogen atoms one protoneach and one oxygen atom 8 protons and 8 neutrons for a totalof 18 nucleons. Cholesterol is a small molecule with a molecular mass of 387 gmol or387 Da. A mole is the molecular weight of asubstance. Add that to a container and bring the volume to five liters with water.
The gram molecular mass of water is 18 grams per mole.
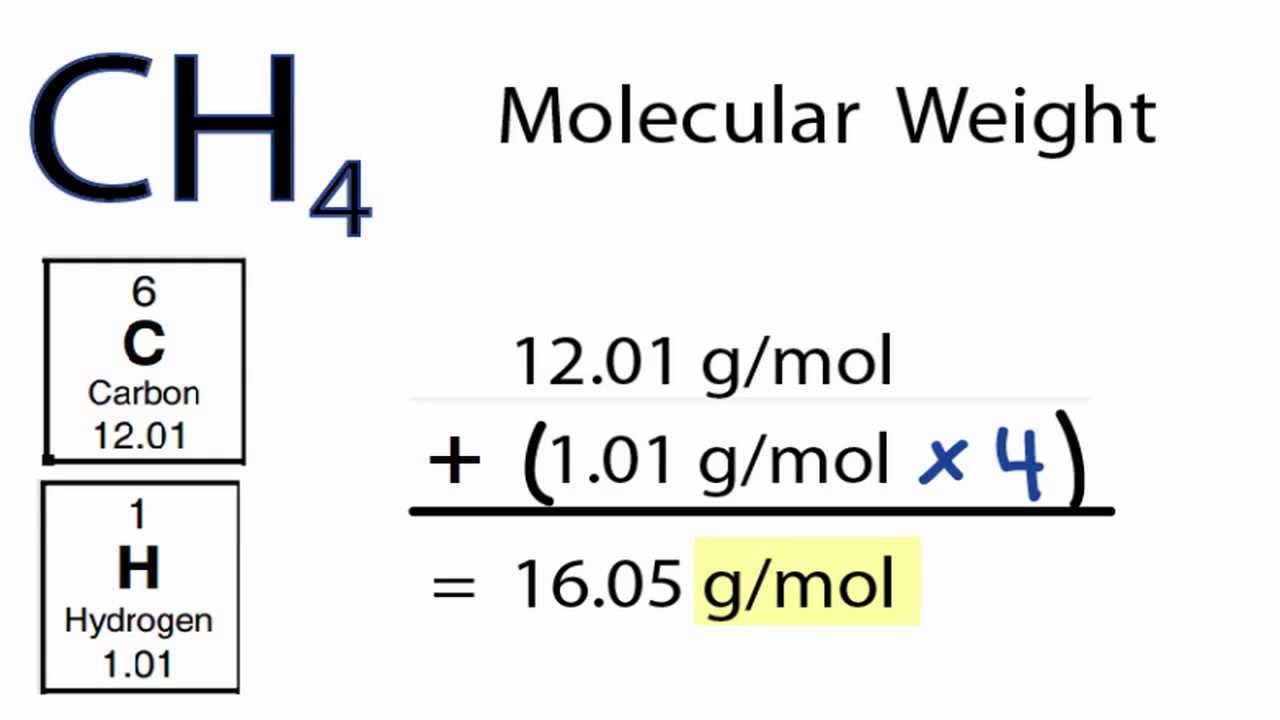
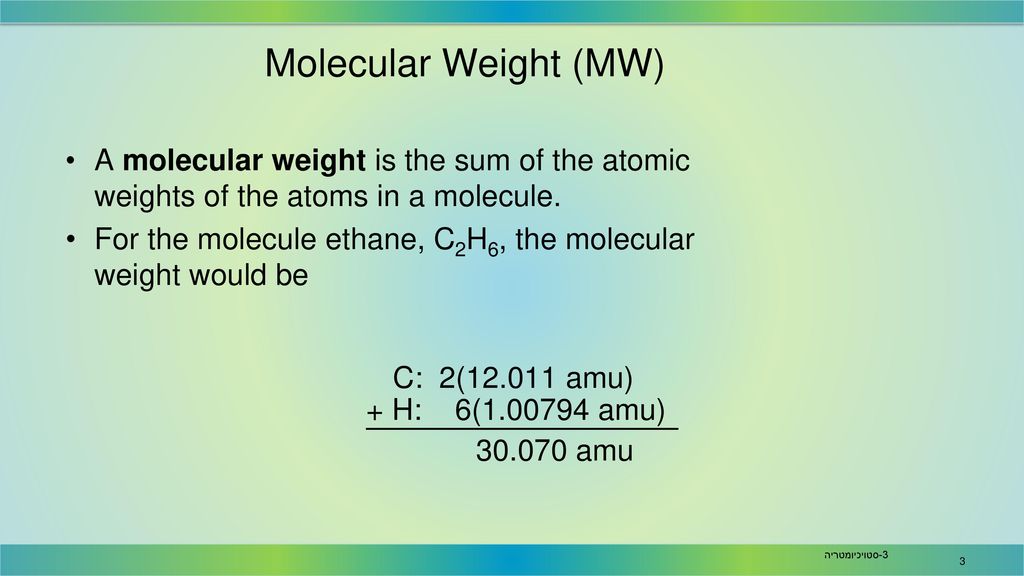
Hydrogen has a molar mass of 1008 grams per mole 008 grams per mole. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. For example the mean molecular weight of water is 18015 atomic mass units amu so one mole of water weight 18015 grams. Carbon is out there within the atomic type. Sowe want to end up with grams litre from moleslitre. Chemical Compound Formulas In general the formal definition of the molecular weight is the average mass of the chemical compound which is compared to the 112 the mass of the carbon 12.
 Source: slideplayer.com
Source: slideplayer.com
It is determined by the sum of the atomic weight of the constituent atoms. Finding molar mass starts with units of grams per mole gmol. So carbon has a molar mass of 1201 grams per mole and now we can think about hydrogen in the same way. Since there are 2 hydrogens in the molecule the total weight of hydrogen in water is 2 times 100794 or 201588. For example the mean molecular weight of water is 18015 atomic mass units amu so one mole of water weight 18015 grams.
 Source: youtube.com
Source: youtube.com
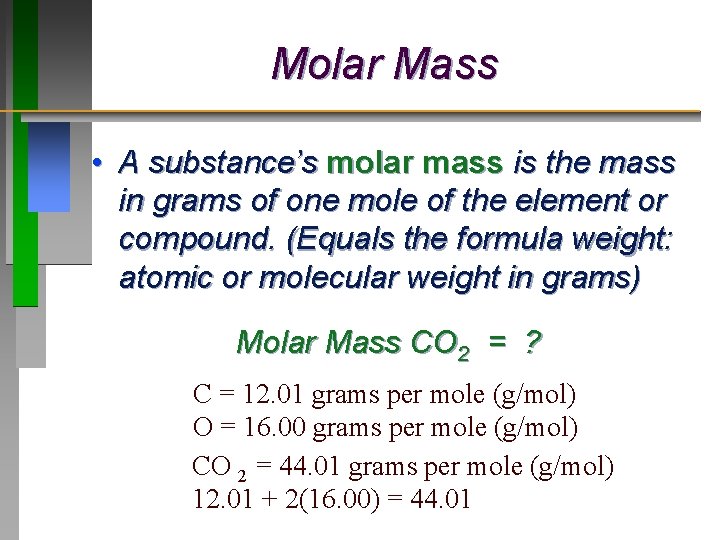
There are four atoms of O Oxygen therefore 416 64 gmol. Sowe want to end up with grams litre from moleslitre. Grams per mole to Kilograms per mole gmol to kgmol converter. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance.
 Source: slideplayer.com
Source: slideplayer.com
One molecular hydrogen molecular atom has molecular mass of 1 Da so 1 Da 1 gmol. There are 2 ways used for finding molar mass and calculating molecular weight. Kilogram per mole kgmol 10 -3. Molarity x molar mass moleslitre x grams mole the moles cancel leaving. The weight of one mole is 84 grams so the weight of 7 grams is 588 grams or 0588 kilograms.
 Source: youtube.com
Source: youtube.com
What do we multiply moleslitre by to get gramslitre. 1 Gram per mole gmol is equal 0001 Kilogram per. The French physicist Jean Perrin called the number of units in the sum of one mole Avogadro a few years later. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. Finding molar mass starts with units of grams per mole gmol.
 Source: slideplayer.com
Source: slideplayer.com
The formula mass and molecular mass of water H 2 O are one and the same while the formula and molecular mass of glucose are different from each other. How many moles are in 1 gram. Cholesterol is a small molecule with a molecular mass of 387 gmol or387 Da. There are 2 ways used for finding molar mass and calculating molecular weight. For hydrogen the atomic weight is 100794 and for oxygen it is 159994.
 Source: youtube.com
Source: youtube.com
For example the mean molecular weight of water is 18015 atomic mass units amu so one mole of water weight 18015 grams. How do you convert micro-moles per gram to milligrams per kilogram. This isbecause a water molecule contains two hydrogen atoms one protoneach and one oxygen atom 8 protons and 8 neutrons for a totalof 18 nucleons. How many moles are in a Dalton. Since there are 2 hydrogens in the molecule the total weight of hydrogen in water is 2 times 100794 or 201588.
 Source: slidetodoc.com
Source: slidetodoc.com
Sowe want to end up with grams litre from moleslitre. Finding molar mass starts with units of grams per mole gmol. The French physicist Jean Perrin called the number of units in the sum of one mole Avogadro a few years later. For example one mole of water molecules contains 6022140758 x 1023 molecules. Another property of Avogadros number is that the mass of one mole of a substance is equal to that substances molecular weight.
 Source: slideplayer.com
Source: slideplayer.com
The formula mass and molecular mass of water H 2 O are one and the same while the formula and molecular mass of glucose are different from each other. For example the mean molecular weight of water is 18015 atomic mass units amu so one mole of water weight 18015 grams. Molecular weight formula helps the molecular mass calculator to find molar mass grams per moles and molecular weight. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. The formula mass formula weight of glucose is 30 either no units or else grams per mole while the molecular mass molecular weight is 180156 gmol.
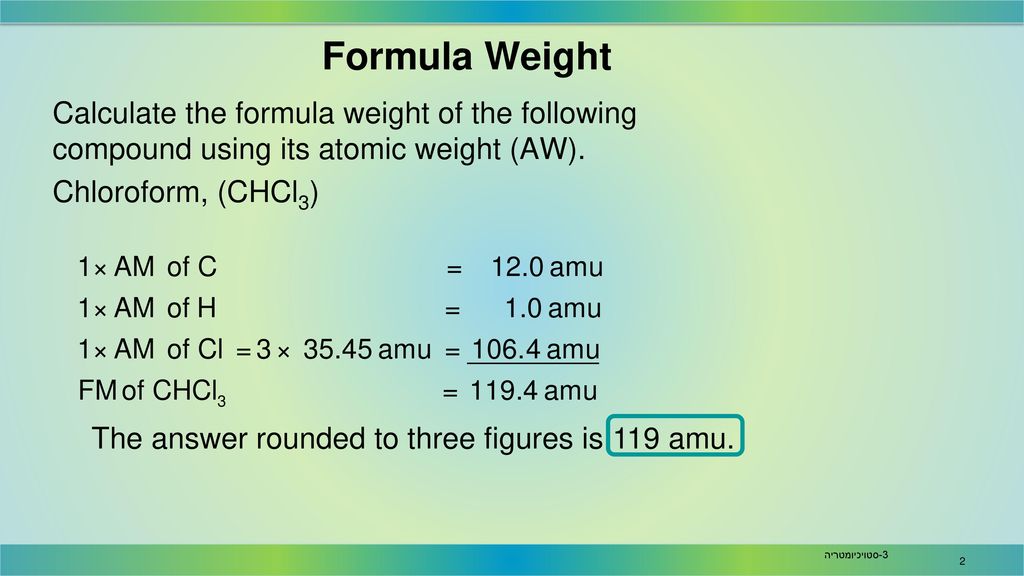 Source: slideplayer.com
Source: slideplayer.com
There are four atoms of O Oxygen therefore 416 64 gmol. Sowe want to end up with grams litre from moleslitre. How many moles are in a Dalton. So carbon has a molar mass of 1201 grams per mole and now we can think about hydrogen in the same way. There are 2 ways used for finding molar mass and calculating molecular weight.
 Source: study.com
Source: study.com
To complete this calculation you. The formula mass formula weight of glucose is 30 either no units or else grams per mole while the molecular mass molecular weight is 180156 gmol. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. There is only 1 oxygen so the total weight of oxygen is 159994. 5 rows By the help of this simple calculation we find that the mass of one mole of NaCl compound is.
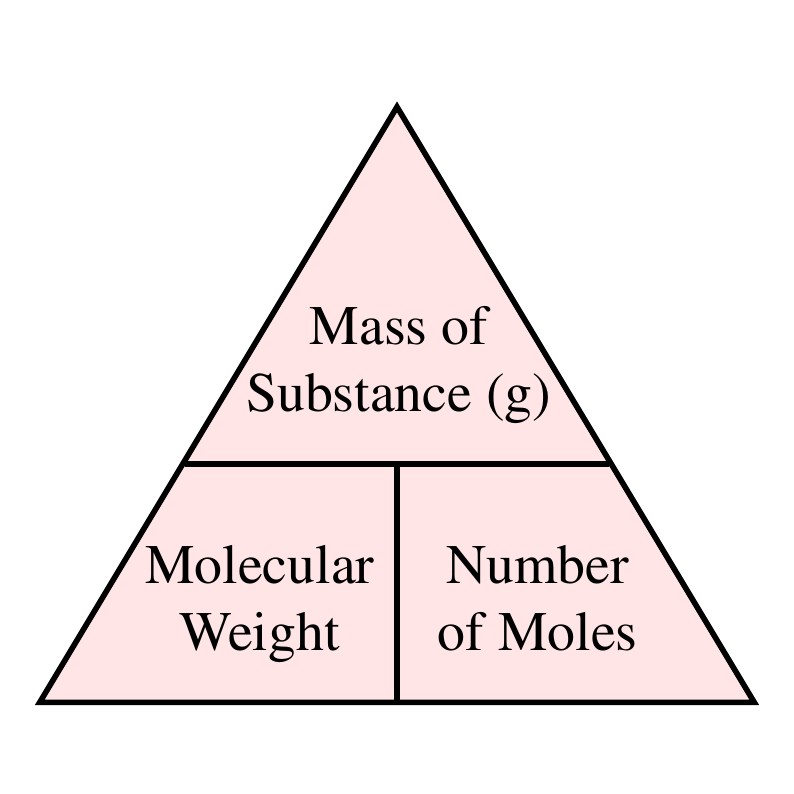 Source: ncl.ac.uk
Source: ncl.ac.uk
Grams per mole to Kilograms per mole gmol to kgmol converter. What do we multiply moleslitre by to get gramslitre. Molarity x molar mass moleslitre x grams mole the moles cancel leaving. For example the average mass of one molecule of water is about 180153 daltons and one mole of water is about 180153 grams. Since there are 2 hydrogens in the molecule the total weight of hydrogen in water is 2 times 100794 or 201588.
 Source: slideplayer.com
Source: slideplayer.com
Carbon is out there within the atomic type. A common request on this site is to convert grams to moles. The molecular weight of barium Ba is 1373 grams per mole The molecular weight of oxygen O is 16 grams per mole The molecular weight of hydrogen H is 100 grams per mole. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. So carbon has a molar mass of 1201 grams per mole and now we can think about hydrogen in the same way.
 Source: slideplayer.com
Source: slideplayer.com
The density of one mole in grams is the weight in atomic mass units of that element. The molecular weight of barium Ba is 1373 grams per mole The molecular weight of oxygen O is 16 grams per mole The molecular weight of hydrogen H is 100 grams per mole. Molarity x molar mass moleslitre x grams mole the moles cancel leaving. Oxygen we can see from our periodic table of elements it has a molar mass of 1600 grams per mole. There are four atoms of O Oxygen therefore 416 64 gmol.
 Source: khanacademy.org
Source: khanacademy.org
Carbon is out there within the atomic type. Finding molar mass starts with units of grams per mole gmol. Finally we add up the weights of all the atoms to get the total molecular weight of water 180153 grams per mole. Molarity x molar mass moleslitre x grams mole the moles cancel leaving. For example one mole of water molecules contains 6022140758 x 1023 molecules.
 Source: slideshare.net
Source: slideshare.net
The conversion rate is 350 grams dividedby 46 gram per mole. Oxygen we can see from our periodic table of elements it has a molar mass of 1600 grams per mole. For hydrogen the atomic weight is 100794 and for oxygen it is 159994. The weight of one mole is 84 grams so the weight of 7 grams is 588 grams or 0588 kilograms. Multiply by the.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title molecular weight in grams per mole by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






