Your Mole of oxygen gas to grams images are available. Mole of oxygen gas to grams are a topic that is being searched for and liked by netizens now. You can Download the Mole of oxygen gas to grams files here. Download all free photos.
If you’re looking for mole of oxygen gas to grams images information related to the mole of oxygen gas to grams interest, you have pay a visit to the ideal site. Our website frequently provides you with hints for downloading the maximum quality video and image content, please kindly hunt and find more enlightening video articles and graphics that match your interests.
Mole Of Oxygen Gas To Grams. 1 cubic meter of Oxygen weighs 1429 kilograms kg 1 cubic inch of Oxygen weighs 0000826014 ounce oz Oxygen weighs 0001429 gram per cubic centimeter or 1429 kilogram per cubic meter ie. 1 mole is equal to 1 moles O2 or 319988 grams. Note that rounding errors may occur so always check the results. 9 moles Oxygen to grams.
 Calculate The Number Of Molecules In 4 G Of Oxygen Youtube From youtube.com
Calculate The Number Of Molecules In 4 G Of Oxygen Youtube From youtube.com
How many atoms are present in 16 grams of oxygen. Thus the number of molecules in 16 gm or 05 mole of oxygen gas are 05 x 6023 x 1023 3012 x 1023 molecules. How many grams are in a mole of water. 32 grams of oxygen gas is 1 mole. B Molecular weight of water H2 Oin grams 2 1618 gone mole of water. How many moles of oxygen o2 are in 500 g of oxygen.
Complete reaction with 0850 moles of oxygen gas.
How many grams are in a mole of water. If we total up the gram amounts of each element in the water molecule 15998gmol 21008gmol we get the molar mass of water 18014gmol. 4 moles Oxygen to grams 639976 grams. Divide the amount you have by the molar weight to obtain the number of moles in the sample. Density of oxygen is equal to 1429 kgm³. The number of molecules in 16 gm of oxygen are 05 1632 moles.
 Source: in.pinterest.com
Source: in.pinterest.com
So the answer would be 23584-22347grams divided. 1 Oxygen atom has a mass of 16 grams thus diatomic oxygen weights twice as much- 32 grams. 7 moles Oxygen to grams 1119958 grams. 1 10 -6. One mole of.
 Source: youtube.com
Source: youtube.com
How many atoms are present in 16 grams of oxygen. 1 cubic meter of Oxygen weighs 1429 kilograms kg 1 cubic inch of Oxygen weighs 0000826014 ounce oz Oxygen weighs 0001429 gram per cubic centimeter or 1429 kilogram per cubic meter ie. If we total up the gram amounts of each element in the water molecule 15998gmol 21008gmol we get the molar mass of water 18014gmol. How many moles of oxygen o2 are in 500 g of oxygen. Hope you understood well Anusha Bhargava.
 Source: youtube.com
Source: youtube.com
The number of molecules in 16 gm of oxygen are 05 1632 moles. Divide the amount you have by the molar weight to obtain the number of moles in the sample. Molecular weight of O2 or grams The SI base unit for amount of substance is the mole. 5 moles Oxygen to grams 79997 grams. Mass-to-Mass Conversions Grams A Moles A Moles B Grams B Conversion factor.
 Source: youtube.com
Source: youtube.com
What is the other gas if it could be any of the following. 4 moles Oxygen to grams 639976 grams. Thus the number of molecules in 16 gm or 05 mole of oxygen gas are 05 x 6023 x 1023 3012 x 1023 molecules. 1 mole is equal to 1 moles Oxygen or 159994 grams. Divide the amount you have by the molar weight to obtain the number of moles in the sample.
 Source: youtube.com
Source: youtube.com
How many atoms are present in 16 grams of oxygen. You can view more details on each measurement unit. A Molecular weight of oxygen in grams 32 g of oxygen one mole of oxygen gas. 48 grams of oxygen gas is 13248 mole. One mole of.
 Source: youtube.com
Source: youtube.com
Molecular weight of O2 or grams The SI base unit for amount of substance is the mole. You can view more details on each measurement unit. Thus the number of molecules in 16 gm or 05 mole of oxygen gas are 05 x 6023 x 1023 3012 x 1023 molecules. How many atoms are present in 16 grams of oxygen. Mass of P2O5 P 2 O 5 is 0290 g.
 Source: m.youtube.com
Source: m.youtube.com
H 2O2 3402 gmol Ex. 32 grams of oxygen gas is 1 mole. How many moles of oxygen o2 are in 500 g of oxygen. Gram atomic mass is the mass in grams of one mole of atoms in a monatomic chemical element. One mole of atoms of oxygen has a mass of 16 g as 16 is the atomic.
 Source: pinterest.com
Source: pinterest.com
6 moles Oxygen to grams 959964 grams. 1 grams of oxygen gas is 132 mole. 1 Oxygen atom has a mass of 16 grams thus diatomic oxygen weights twice as much- 32 grams. Divide the amount you have by the molar weight to obtain the number of moles in the sample. 2 moles Oxygen to grams 319988 grams.
 Source: youtube.com
Source: youtube.com
This online calculator converts grams to liters and liters to grams given a gas formula. In other words 1 mole of oxygen would contain molecules. Note that rounding errors may occur so always check the results. At 0C 32F or 27315K at standard atmospheric pressure. 32 grams of oxygen gas is 1 mole.
 Source: pinterest.com
Source: pinterest.com
N 2 moles Thus according to the molar ratio we should have half the amount of moles that is one mole of diatmoic oxygen. 1 mole is equal to 1 moles O2 or 319988 grams. So the answer would be 23584-22347grams divided. 24 grams 32 gramsmol 075 moles. 5 moles Oxygen to grams 79997 grams.
 Source: pinterest.com
Source: pinterest.com
Divide the amount you have by the molar weight to obtain the number of moles in the sample. The rate of effusion of oxygen gas O2 Molar Mass 32 gramsmole to an unknown gas is 0935. The number of molecules in 16 gm of oxygen are 05 1632 moles. 24 grams 32 gramsmol 075 moles. Use molar mass of A Use mole ratio from the balanced equation Use molar mass of B molar mass N 2H4 3205gmol.
 Source: youtube.com
Source: youtube.com
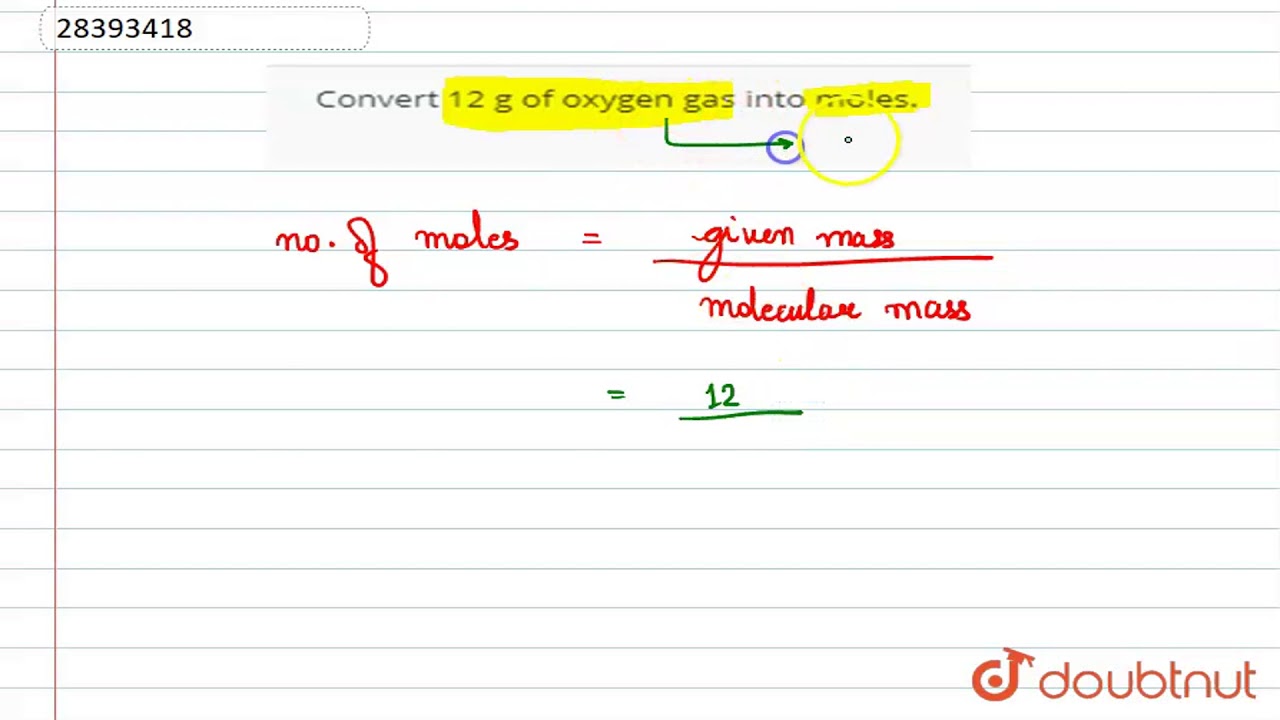
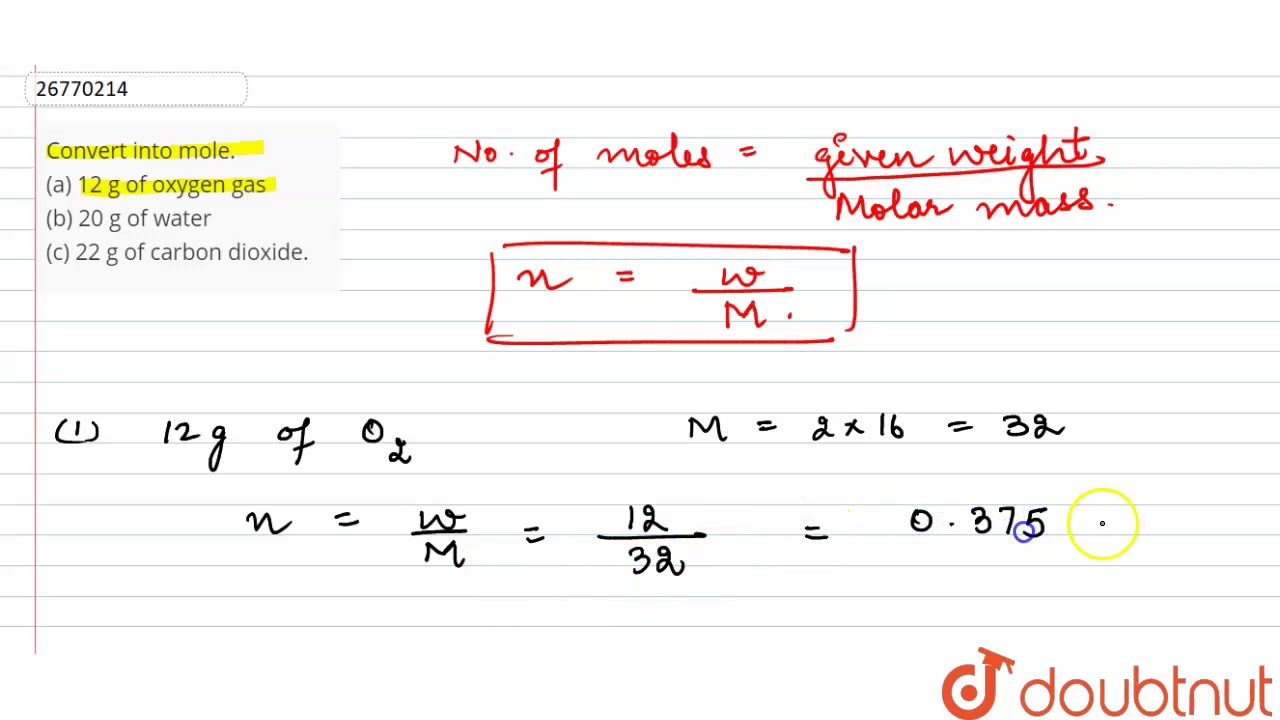
Given that 12 g of oxygen gas. Oxygen gasA few things to consider when finding the molar mass for O2- make sure you have the correct chem. From the given mass of P2O5 P 2 O 5 the number. 1 grams of oxygen gas is 132 mole. We have to find the moles of oxygen.
 Source: youtube.com
Source: youtube.com
The atomic mass of an element expressed in grams is called. 8 moles Oxygen to grams 1279952 grams. Hope you understood well Anusha Bhargava. 1 gram of oxygen gas 132 mole. Likewise how many grams is 1667 moles of oxygen.
 Source: youtube.com
Source: youtube.com
24 grams 32 gramsmol 075 moles. You can view more details on each measurement unit. 2 moles Oxygen to grams 319988 grams. You can view more details on each measurement unit. The answer is 319988.
 Source: youtube.com
Source: youtube.com
Molecular weight of O2 or grams The SI base unit for amount of substance is the mole. What is the other gas if it could be any of the following. Complete reaction with 0850 moles of oxygen gas. 8 moles Oxygen to grams 1279952 grams. Thus 12 g of oxygen321 120375 moles.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title mole of oxygen gas to grams by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






