Your Mole of nacl equals how many grams images are available in this site. Mole of nacl equals how many grams are a topic that is being searched for and liked by netizens now. You can Get the Mole of nacl equals how many grams files here. Download all royalty-free photos and vectors.
If you’re searching for mole of nacl equals how many grams pictures information related to the mole of nacl equals how many grams keyword, you have pay a visit to the ideal site. Our site always provides you with hints for seeking the maximum quality video and image content, please kindly hunt and locate more informative video content and graphics that fit your interests.
Mole Of Nacl Equals How Many Grams. 1 mole is equal to 1 moles NaCl or 5844277 grams. Do a quick conversion. 1 mole is equal to 1 moles 2 NaCl or 6044277 grams. Type in your own numbers in the form to convert the units.
 How To Convert Grams Of Nacl To Moles Of Nacl Youtube From youtube.com
How To Convert Grams Of Nacl To Moles Of Nacl Youtube From youtube.com
To go from grams to moles divide the grams by the molar mass. The SI base unit for quantity of substance is the mole. You have 12 grams which equals 12585 021 moles. 3 moles NaCl to grams 17532831 grams. Use this page to learn how to convert between moles NaCl and gram. The SI base unit for amount of substance is the mole.
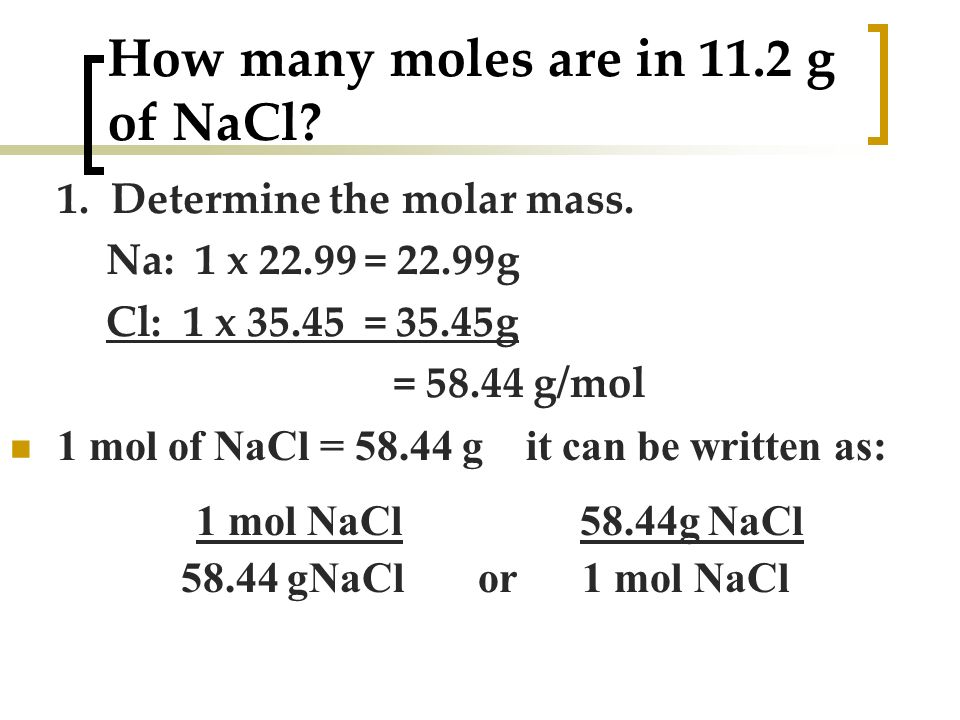
How many moles are in NaCl.
This compound is also known as Sodium Chloride. 4 moles NaCl come grams 23377108 grams. - Then the molecular weight of the sodium chloride NaCl 23 355 585 g. 1 moles NaCl to grams 5844277 grams. Molar mass of NaCl atomic mass of Na 2299 amu the atomic mass of Cl 3545 amu 2299 3545 5844 amu One mol of NaCl 602 x10 23 formulas has a mass of 5844 g. This means that the mass of one mole of sodium chloride is equal to.
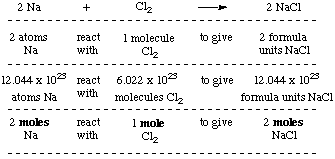 Source: chemiris.labs.brocku.ca
Source: chemiris.labs.brocku.ca
Also like and subscribe to keep the videos coming. This means that the mass of one mole of sodium chloride is equal to. You have 12 grams which equals 12585 021 moles. 1 grams NaCl is equal to 0017110756386119 mole. 3 moles NaCl to grams 17532831 grams.

We assume you are converting between moles NaCl and gram. Use this page to learn how to convert between grams 2 NaCl and mole. Note that rounding errors may occur so always check the results. 1 grams NaCl 0017110756386119 mole using the molecular weight calculator and the molar mass of NaCl. Use this page to learn how to convert between moles 2 NaCl and gram.
 Source: brainly.com
Source: brainly.com
Also like and subscribe to keep the videos coming. To go from grams to moles divide the grams by the molar mass. 4 moles NaCl to grams 23377108 grams. The molecular formula for NaCl is NaCl. 2 moles NaCl to grams 11688554 grams.
 Source: slideplayer.com
Source: slideplayer.com
Use this page to learn how to convert between moles NaCl and gram. Rounding to one decimal place the mass of one mole of sodium Na is 230 grams and that of chlorine Cl is 355 grams so one mole of NaCl has a mass of 585 grams. The molecular formula for NaCl is NaCl. You find this by taking the molar mass of Na which is 2299 and Cl which is 3545 and you add them together then divide by 100. NaCl is an ionic compound so there are no molecules only formula units.
 Source: discoveryexpresskids.com
Source: discoveryexpresskids.com
To convert between grams and moles you would use the substances molar mass. How do you calculate 1 mole of NaCl. 2 moles NaCl to grams 11688554 grams. Molar mass of NaCl atomic mass of Na 2299 amu the atomic mass of Cl 3545 amu 2299 3545 5844 amu One mol of NaCl 602 x10 23 formulas has a mass of 5844 g. We assume you are converting between moles NaCl and gram.
 Source: youtube.com
Source: youtube.com
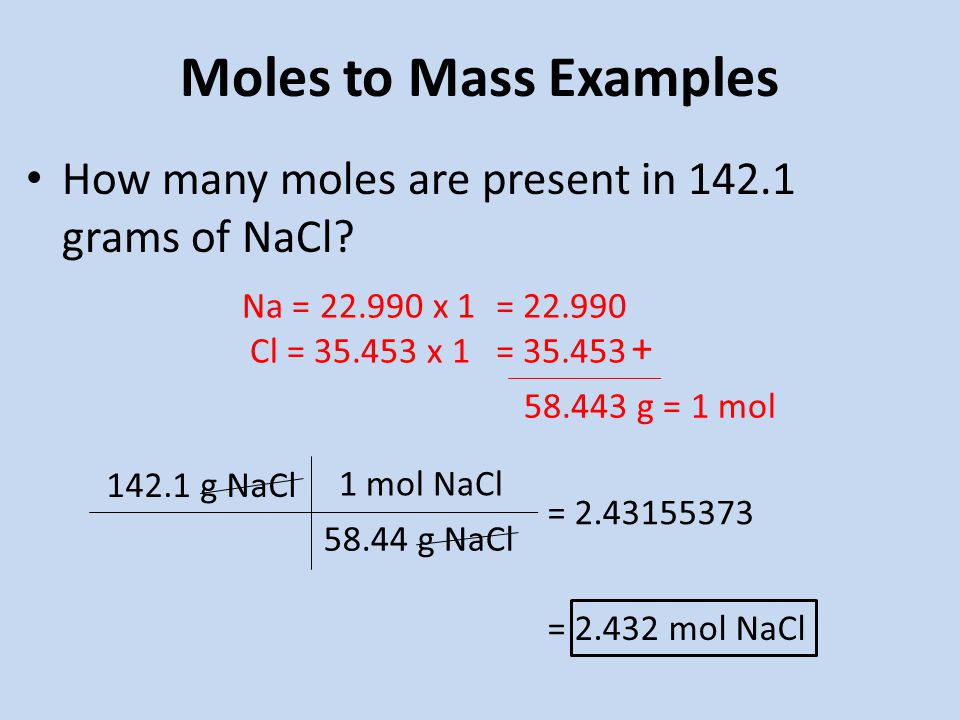
There is 171 moles in 100 grams of NaCl. Use this page to learn how to convert between moles NaCl and gram. 1 mole 60221023 6022 10 23 atoms molecules protons etc. We assume you are converting between moles NaCl and gram. The SI base unit for amount of substance is the mole.
 Source: slideplayer.com
Source: slideplayer.com
1 mole is equal to 1 moles NaCl or 5844277 grams. To convert between grams and moles you would use the substances molar mass. The SI base unit for amount of substance is the mole. 1 grams NaCl 0017110756386119 mole using the molecular weight calculator and the molar mass of NaCl. - The atomic weight of the sodium is 23 and the atomic weight of the chlorine is 355.
 Source: youtube.com
Source: youtube.com
124 1024 formula units. Use this page to learn how to convert between moles NaCl and gram. Use this page to learn how to convert between moles NaCl and gram. 1 grams NaCl is equal to 0017110756386119 mole. The SI base unit for amount of substance is the mole.
 Source: clutchprep.com
Source: clutchprep.com
This compound is also known as Sodium Chloride. Molar mass of NaCl atomic mass of Na 2299 amu the atomic mass of Cl 3545 amu 2299 3545 5844 amu One mol of NaCl 602 x10 23 formulas has a mass of 5844 g. Also like and subscribe to keep the videos coming. Note that rounding errors may occur so always check the results. The SI base unit for amount of substance is the mole.
 Source: youtube.com
Source: youtube.com
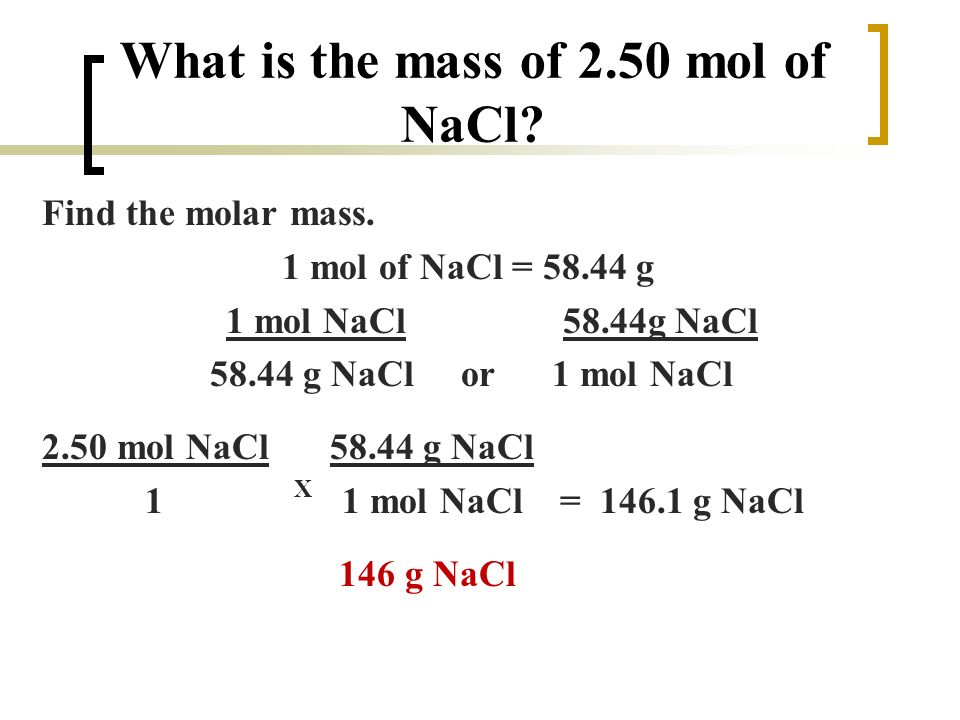
To convert between grams and moles you would use the substances molar mass. What number of moles of NaCl are wanted to arrange 150 liters of 0250 M answer. 1 grams NaCl 0017110756386119 mole using the molecular weight calculator and the molar mass of NaCl. You have 12 grams which equals 12585 021 moles. M M NaCl 5844247 g mol1.
 Source: clutchprep.com
Source: clutchprep.com
To convert between grams and moles you would use the substances molar mass. To go from grams to moles divide the grams by the molar mass. 124 1024 formula units. 1 mole is the same as 1 moles NaCl or 5844277 grams. 2 moles NaCl come grams 11688554 grams.
 Source: slideplayer.com
Source: slideplayer.com
2 moles NaCl come grams 11688554 grams. 1 mole is equal to 1 moles Oxygen or 159994 grams. 1 moles NaCl to grams 5844277 grams. M M NaCl 2298977 g mol1 354527 g mol1. Use this page to learn how to convert between moles NaCl and gram.
 Source: youtube.com
Source: youtube.com
Formula units NaCl 120 g NaCl 1 mol NaCl 5844 g NaCl 602 1023 formula units 1 mol NaCl. The SI base unit for amount of substance is the mole. You find this by taking the molar mass of Na which is 2299 and Cl which is 3545 and you add them together then divide by 100. Do a quick conversion. What number of moles of NaCl are wanted to arrange 150 liters of 0250 M answer.
 Source: slideplayer.com
Source: slideplayer.com
Use this page to learn how to convert between moles NaCl and gram. The SI base unit for amount of substance is the mole. NaCl is an ionic compound so there are no molecules only formula units. Note that rounding errors may occur so always check the results. To go from grams to moles divide the grams by the molar mass.
 Source: slideplayer.com
Source: slideplayer.com
The SI base unit for amount of substance is the mole. You have 12 grams which equals 12585 021 moles. Quick conversion chart of moles NaCl to grams. - The atomic weight of the sodium is 23 and the atomic weight of the chlorine is 355. The SI base unit for amount of substance is the mole.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title mole of nacl equals how many grams by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.