Your Mole of carbon dioxide contains images are available in this site. Mole of carbon dioxide contains are a topic that is being searched for and liked by netizens today. You can Get the Mole of carbon dioxide contains files here. Get all free vectors.
If you’re searching for mole of carbon dioxide contains images information related to the mole of carbon dioxide contains keyword, you have come to the ideal site. Our site frequently provides you with suggestions for seeking the highest quality video and image content, please kindly hunt and find more informative video content and graphics that fit your interests.
Mole Of Carbon Dioxide Contains. Hence 5 mole of carbon dioxide consists of 301 x1024 molecules. 8 g O atoms 1 mol O atoms16 g O atoms 1 mol CO22 mol O atoms 025 mol CO2. Carbon dioxide is not a mixture of carbon and oxygen gas. 1g of oxygen 132 mole of carbon dioxide.
 The Mole Concept Avogadros Constant Mr Carson S Science Page From sites.google.com
The Mole Concept Avogadros Constant Mr Carson S Science Page From sites.google.com
One mole of C 60210 23 atoms of carbon. 60210 23 is Avogadros number and represents number of atomsmolecules present in one mole of substance. 1420 moles CO₂. 8 g O atoms 1 mol O atoms16 g O atoms 1 mol CO22 mol O atoms 025 mol CO2. Each molecule of CO2 contains one atom of carbon and two atoms of oxygen. 24 1022 molecules CO2 1 mole CO2 6022 1023 molecules CO2.
The mole is represented by Avogadros number which is 6022 10 23 atoms or molecules per mol.
We know it has the formula CO2 and this tells us that. Mass of one Carbon mole 12 g. What is the molar mass of carbon dioxide. Carbon dioxide has a chemical formula CO2 which means it has a molar mass of 1201 3200 4401 g mol. 5 How many moles are in 10 grams of co2. A carbon dioxide molecule which has the formula CO2 contains one carbon atom and two oxygen atoms.
 Source: youtube.com
Source: youtube.com
03636 6022 x 1023 molecules mol 21895 x 1023 molecules of carbon dioxide. Molecular mass of oxygen 16. 120108 gmol 2159994 gmol 440096 gmol B. Thus 1 mol of neon contains 1 mol of atoms. How many moles of carbon dioxide are in your sample.
 Source: sites.google.com
Source: sites.google.com
As one mole of carbon dioxide contains 602 10 23 atoms of carbon half mole of carbon dioxide will contain half of the 602 10 23 atoms of carbon and that will be equal to 301 10 23 carbon atoms. Therefore to calculate the amount of CO2 produced from a gallon of gasoline the weight of the carbon in the gasoline is multiplied by 4412 or 37. Molecular mass of oxygen 16. 120108 gmol 2159994 gmol 440096 gmol B. 8 g of oxygen 81 32 025 mol.
 Source: pinterest.com
Source: pinterest.com
How many moles are present in 16 grams of carbon dioxide. 1 mole of carbon dioxide consist of 6023 x1023 molecules. Two moles of O 260210 23120410 23 atoms of oxygen. One mole of C 60210 23 atoms of carbon. It is a compound made of molecules.
 Source: toppr.com
Source: toppr.com
Therefore there are 36132 times 10 to the 24th power atoms of oxygen in 3 moles of pure carbon dioxide. 1 mole of carbon dioxide consist of 6023 x1023 molecules. A mole of chlorine gas contains 2 x 602 x 1023 molecules. 1 mole of carbon atoms per mole of carbon dioxide 602 x 1023 carbon atoms. How many moles are present in 16 grams of carbon dioxide.
 Source: slideplayer.com
Source: slideplayer.com
120108 gmol 2159994 gmol 440096 gmol B. A carbon atom has a weight of 12 and each oxygen atom has a weight of 16 giving each single molecule of CO2 an atomic weight of 44 12 from carbon and 32 from oxygen. A mole of carbon dioxide CO2 contains two moles of oxygen atoms. 44 grams is a mole of carbon dioxide and there is one carbon atom in CO so there is one mole of carbon. 1 mole of carbon atoms per mole of carbon dioxide 602 x 1023 carbon atoms.
 Source: pinterest.com
Source: pinterest.com
Since one mole of CO2 contains one mole carbon atoms therefore 06 moles of carbon dioxide will contain 06 moles of carbon. 003985moles CO2 4401 g 1mole CO2. 1 mole carbon dioxide contains 602 x 1023 molecules. A carbon atom has a weight of 12 and each oxygen atom has a weight of 16 giving each single molecule of CO2 an atomic weight of 44 12 from carbon and 32 from oxygen. Two moles of O 260210 23120410 23 atoms of oxygen.

Molecular mass of CO 2 12 16 X 2 12 32. Mass of one Oxygen mole 16 g. 1420 moles CO₂. 1g of oxygen 132 mole of carbon dioxide. We assume you are converting between moles CO2 and gram.
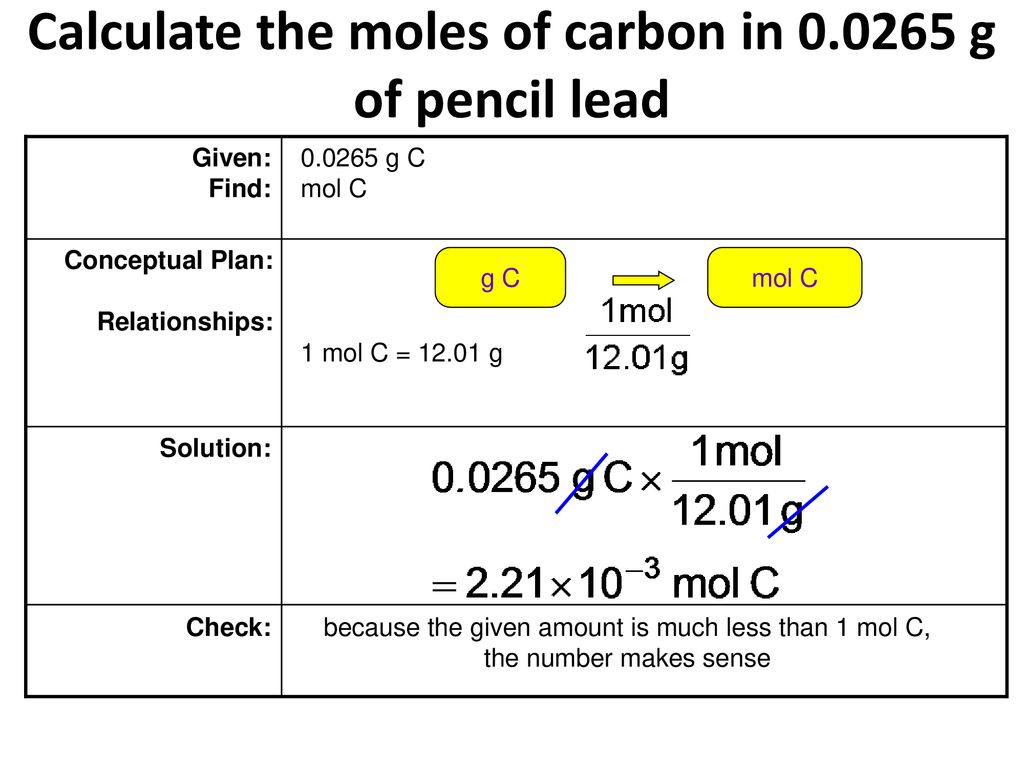 Source: slideplayer.com
Source: slideplayer.com
8 g of oxygen 81 32 025 mol. Molecular mass of oxygen 16. A mole consists of the number of particles in exactly 12g of naturally occurring carbon. One mole of C 60210 23 atoms of carbon. This information is contained in the subscripts after each element.
 Source: co.pinterest.com
Source: co.pinterest.com
A carbon dioxide molecule which has the formula CO2 contains one carbon atom and two oxygen atoms. A carbon atom has a weight of 12 and each oxygen atom has a weight of 16 giving each single molecule of CO2 an atomic weight of 44 12 from carbon and 32 from oxygen. Carbon dioxide has a chemical formula CO2 which means it has a molar mass of 1201 3200 4401 g mol. A molecule of sucrose C12H22O11 has 12 carbon atoms 22 hydrogen atoms and 11 oxygen atoms. A mole of carbon dioxide CO2 contains two moles of oxygen atoms.
 Source: in.pinterest.com
Source: in.pinterest.com
How many carbon atoms are in your sample. Two O and one C 688 22 thus one mole of CO2 has 22 6022 1023 electrons and 227 mol has 227 22 6022 1023 electrons 3011 1023 electrons. 685 g x 1 mole440096 g 0156 moles. We know it has the formula CO2 and this tells us that. 1 mole carbon dioxide contains 602 x 1023 molecules.

5 How many moles are in 10 grams of co2. Each molecule of CO2 contains one atom of carbon and two atoms of oxygen. A mole of chlorine gas contains 2 x 602 x 1023 molecules. The answer is 0022722366761722. Therefore there are 36132 times 10 to the 24th power atoms of oxygen in 3 moles of pure carbon dioxide.

1 mole of carbon dioxide consist of 6023 x1023 molecules. A mole of oxygen gas contains 602 x. 1420 moles CO₂. Molecular mass of CO 2 12 16 X 2 12 32. 8 g of oxygen 81 32 025 mol.

As one mole of carbon dioxide contains 602 10 23 atoms of carbon half mole of carbon dioxide will contain half of the 602 10 23 atoms of carbon and that will be equal to 301 10 23 carbon atoms. Molecular mass of oxygen 16. A sample contains 685 g of carbon dioxide CO 2. 1 mole of carbon atoms per mole of carbon dioxide 602 x 1023 carbon atoms. Two moles of O 260210 23120410 23 atoms of oxygen.

First calculate moles of CO2 first calculate the moles of CO2 10044 227 mol now number of electrons in CO2 are obtained by adding total electrons in each of the three atoms ie. 1 mol of carbon dioxide CO 2 contains 3 mol of atoms. We know it has the formula CO2 and this tells us that. 685 g x 1 mole440096 g 0156 moles. How many carbon atoms are in your sample.
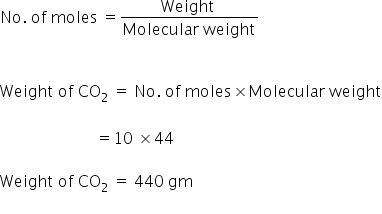 Source: topperlearning.com
Source: topperlearning.com
The provided mass of glycine 28 g is a bit more than one-third the molar mass 75 gmol so we would expect the computed result to be a bit greater than one-third of a mole 033 mol. How many molecules are there in 5 moles of CO2. A mole of chlorine gas contains 2 x 602 x 1023 molecules. Carbon dioxide is not a mixture of carbon and oxygen gas. Molecular mass of CO 2 12 16 X 2 12 32.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title mole of carbon dioxide contains by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






