Your Mole of carbon atoms is equal to 14 grams images are ready. Mole of carbon atoms is equal to 14 grams are a topic that is being searched for and liked by netizens now. You can Download the Mole of carbon atoms is equal to 14 grams files here. Find and Download all free images.
If you’re searching for mole of carbon atoms is equal to 14 grams images information linked to the mole of carbon atoms is equal to 14 grams topic, you have pay a visit to the right site. Our site always provides you with hints for downloading the highest quality video and picture content, please kindly hunt and find more enlightening video articles and graphics that fit your interests.
Mole Of Carbon Atoms Is Equal To 14 Grams. The molecular formula for Carbon is C. 348 x 1022 atoms 1 mole 1187g 686 g Sn 602 x 1023 atoms 1 mole 8. We know that Carbon has atomic mass equal to 12g I. How many moles are in 1982 grams of Mg.
 Structure And Bonding Revision Sheet New Aqa Gcse Chemistry Revision Gcse Chemistry Science Notes From pinterest.com
Structure And Bonding Revision Sheet New Aqa Gcse Chemistry Revision Gcse Chemistry Science Notes From pinterest.com
For carbon-12 12C the molar mass is 1 mole 12C 6022 x 1023 atoms 12C 12 g 12C. How many grams is equal to 348 x 1022 atoms of tin. Atoms or moles the si base unit for amount of substance is the mole. We assume you are converting between grams Carbon and mole. The SI base unit for amount of substance is the mole. Also the Avogadro constant is 602 x 1023.
0 2 2 1 0 2 3 1 2 1.
This number is known as Avogadros number. A mole can be defined as the amount of substance that contains the same number of chemical entities atoms ions molecules etc as there are in 12 g of Carbon-12 isotope as defined by the General. One mole of carbon atom weighs 12g. 448 x 1021 atoms 1 mole 243g 0181 g Mg or. The average mass of a single carbon atom measured in amus. Tap card to see definition.
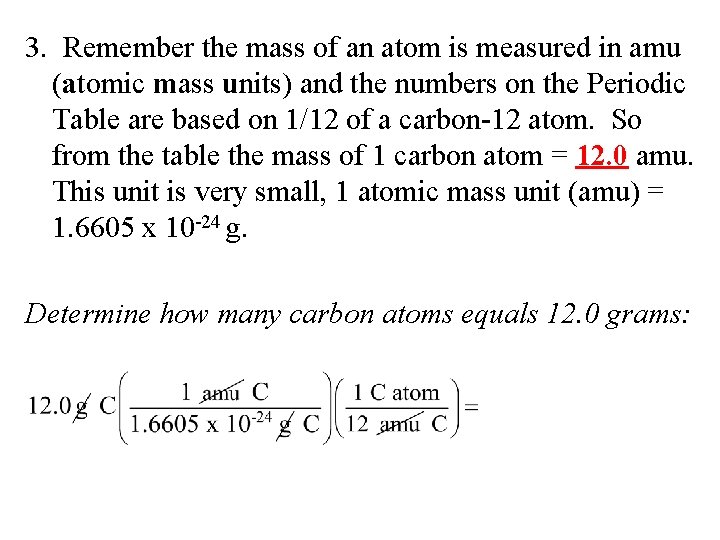 Source: slidetodoc.com
Source: slidetodoc.com
We know that Carbon has atomic mass equal to 12g I. MM of 12C exactly 12 gmol. The numerical value of the mole is defined as being equal to the number of atoms in exactly 12 grams of pure carbon-12 asked Sep 18 2016 in Chemistry by Carmen general-chemistry. How many moles are in 1982 grams of Mg. What is a mole Class 11.
 Source: pinterest.com
Source: pinterest.com
One mole of carbon atom weighs 12g. What would be the mass of 15 moles of H20. The average mass in grams of one mole of carbon atoms is equal to. In a substance the amount of entities present for eg. Mass of a mole of particles mass of 1 particle x 6022 x 1023 The mass of an atom in amu is numerically the same as the mass of one mole of atoms of the element in grams.
 Source: youtube.com
Source: youtube.com
1 mole of nitrogen atoms 14 g. How do you convert particles to grams and grams to particles. Molecular weight of Carbon or mol. So the conversion of carbon from grams to atoms is given by. One mole of S atoms has a mass of 3207 g.
 Source: pinterest.com
Source: pinterest.com
We know that Carbon has atomic mass equal to 12g I. Atoms molecules ions is defined as a moleA mole of any substance is 602210 23 molecules. 7 moles Carbon to grams 840749 grams. The mass of oxygen equal to one mole of oxygen is 15998 grams and the mass of one mole of hydrogen is 1008 g. Molecular weight of Carbon or mol.
 Source: youtube.com
Source: youtube.com
1 grams Carbon is equal to 0083259093974539 mole. 1200 g C-12 1 mol C-12 atoms 6022 1023 atoms The number of particles in 1 mole is called Avogadros Number 60221421 x 1023. We assume you are converting between grams Carbon and mole. 0 2 2 1 0 2 3 a toms of carbon 1 2 g Mass of 1 atom of carbon 6. What is the molar mass of N2.
 Source: slideplayer.com
Source: slideplayer.com
The answer is 120107. How many moles are in 1982 grams of Mg. 4 moles Carbon to grams 480428 grams. Tap card to see definition. 0 2 2 1 0 2 3 a toms of carbon 1 2 g Mass of 1 atom of carbon 6.
 Source: toppr.com
Source: toppr.com
We assume you are converting between grams Carbon and mole. We know that one mole of any substance contains 60225X1023 particles. The value of the mole in precisely 12 grammes of pure carbon-12 is equal to the number of atoms. One mole of any elementcompound has mass equal to its gram atomicmolecular mass. 4 moles Carbon to grams 480428 grams.
 Source: youtube.com
Source: youtube.com
We know that one mole of any substance contains 60225X1023 particles. The answer is 120107. Exactly 12 grams of pure carbon-12 powder is known as one mole. One mole of carbon atom weighs 12g. What is a mole Class 11.
 Source: slideplayer.com
Source: slideplayer.com
Exactly 12 grams of pure carbon-12 powder is known as one mole. Related Links How Many Different Words Can Be Formed By. We know that one mole of any substance contains 60225X1023 particles. The SI base unit for amount of substance is the mole. Tap card to see definition.
 Source: slideplayer.com
Source: slideplayer.com
Click card to see definition. The number of carbon atoms in one amu of carbon. The average mass in grams of one mole of carbon atoms is equal to. Hence mass of 6. Quick conversion chart of moles Carbon to grams.

People found this article helpful. 6022 10 23 atoms 1 mol or. What is the mass of 448 x 1021 atoms of magnesium. The numerical value of the mole is defined as being equal to the number of atoms in exactly 12 grams of. 348 x 1022 atoms 1 mole 1187g 686 g Sn 602 x 1023 atoms 1 mole 8.

We know that one mole of any substance contains 60225X1023 particles. 12C is defined to be exactly 12 g. 8 moles Carbon to. What would be the mass of 15 moles of H20. Click again to see term.
 Source: slideplayer.com
Source: slideplayer.com
The SI base unit for amount of substance is the mole. We know that Carbon has atomic mass equal to 12g I. Now it should be converted in terms of atoms. Use the mole quantity to count formulas by weighing them. Set the amount to 2000 moles of carbon mol C then press Start.
 Source: youtube.com
Source: youtube.com
What is a mole Class 11. Isotope Atomic mass g Molar mass gmol Abundance 12C 1992646 x 10-23 12 exactly 9893. One atom of sulfur has a mass of 3207 amu. 2 moles Carbon to grams 240214 grams. Atoms or moles the si base unit for amount of substance is the mole.
 Source: slidetodoc.com
Source: slidetodoc.com
6022 10 23 atoms 1 mol or. 6022 10 23 atoms 1 mol or. What is the MM of carbon from the periodic table. 0 2 2 1 0 2 3 1 2 1. 1 moles Carbon to grams 120107 grams.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title mole of carbon atoms is equal to 14 grams by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






