Your Molar mass of 18 grams per mole images are ready. Molar mass of 18 grams per mole are a topic that is being searched for and liked by netizens today. You can Download the Molar mass of 18 grams per mole files here. Get all free photos.
If you’re looking for molar mass of 18 grams per mole images information connected with to the molar mass of 18 grams per mole keyword, you have visit the right blog. Our website always gives you suggestions for seeing the maximum quality video and image content, please kindly hunt and locate more enlightening video articles and graphics that fit your interests.
Molar Mass Of 18 Grams Per Mole. 12010714 10079418 1400672 1599945 Percent composition by element. First we need to convert each grams to moles using their molar mass. Moles to Mass Calculation. Into 08 liter L of water dissolve _____.
 Chem Awareness Reaction Stoichiometry Or Chemistry Stoichiometry In 2021 Chemistry Chemical Equation Exothermic Reaction From in.pinterest.com
Chem Awareness Reaction Stoichiometry Or Chemistry Stoichiometry In 2021 Chemistry Chemical Equation Exothermic Reaction From in.pinterest.com
How do you convert moles to liters per molarity. 2 A compound with an empirical formula of C4H4O and a molar mass of 136 grams per mole. At 0C 32F or 27315K at standard atmospheric pressureDefinitions of molecular mass molecular weight molar mass and. Argon weighs 00017838 gram per cubic centimeter or 17838 kilogram per cubic meter ie. Which of the following procedures should you carry out to make a 1 M solution of glucose. Molar mass of molecules can be determined from the chemical formula and molar masses of elements Each H 2O molecule contains 2 H atoms and 1 O atom Each mole of H 2O molecules contains 2 moles of H and 1 mole of O One mole of O atoms corresponds to 159994 g Two moles of H atoms corresponds to 2 x 10079 g Sum molar mass 180152 g H 2O per mole.
The unit of molar mass in the SI system is kilogram per mole.
Density of argon is equal to 17838 kgm³. For example convert 18 grams of water to moles of water. Mass number of moles molar mass. Convert grams Glycine to moles or moles Glycine to grams. N 5988 g 18015 gmol 3324 mol. To calculate the number of moles in a solution given the molarity we multiply the molarity by total volume of the solution in liters.
 Source: slideplayer.com
Source: slideplayer.com
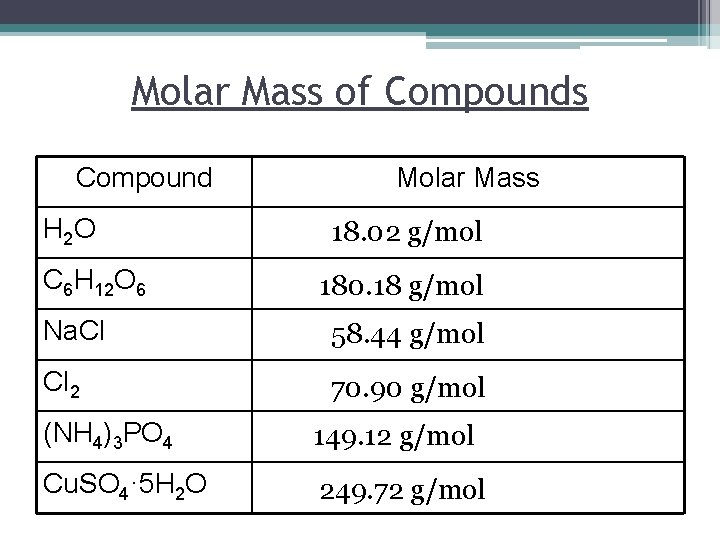
The molar mass is the mass of a given substance chemical element or chemical compound in grams divided by its amount of substance in moleTo determine which of the given options is the molecular formula of glucose we need to calculate the molar mass of each one of them and compare them with the given. It is the amount of grams that is numerically equal to the molecular weight. Molar mass of H2O 1801528 gmol. When we weigh one mole of a substance on a balance this is called a molar mass and has the units gmol grams per mole. Molar mass of NH2CH2COOH 750666 gmol.
 Source: slidetodoc.com
Source: slidetodoc.com
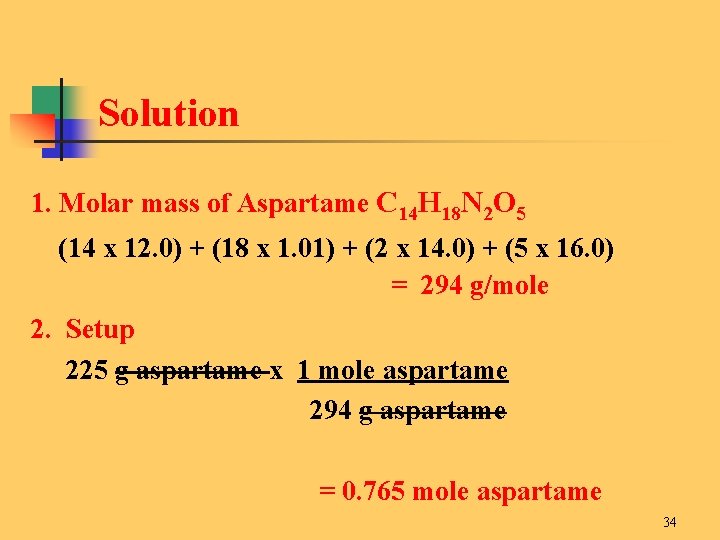
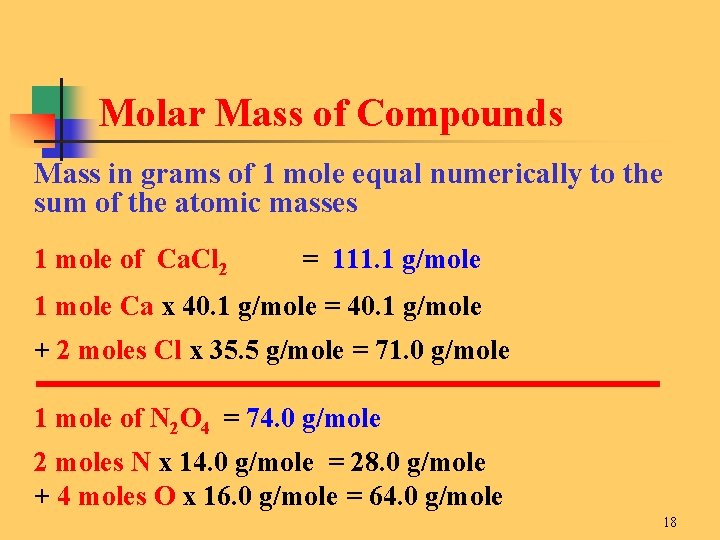
12010714 10079418 1400672 1599945 Percent composition by element. This idea is very critical in chemistry because it is used all the time. For example the molar mass of H20 is 1801 gmol because the molecular weight of H is 1001 g and the molecular weight of O is 1599. That is the molar mass of a substance is the mass in grams per mole of 6022 10 23 atoms molecules or formula units of that substance. 21008 11599 18006 which when rounded for sig figs is 1801 gmol.
 Source: es.pinterest.com
Source: es.pinterest.com
For example the molar mass of H20 is 1801 gmol because the molecular weight of H is 1001 g and the molecular weight of O is 1599. N 5988 g 18015 gmol 3324 mol. Hydrogen- 51 grams 1 mol 101 g 505 moles. Convert grams Aspartame to moles or moles Aspartame to grams. Convert grams Na3PO4 to moles or moles Na3PO4 to grams.
 Source: in.pinterest.com
Source: in.pinterest.com
Therefore the mole is a unit for that physical quantity. This compound is also known as Trisodium Phosphate. This compound is also known as Water or Dihydrogen Monoxide. For a molecule with a weight of 18 the molar mass is 18 grams. Hydrogen- 51 grams 1 mol 101 g 505 moles.
 Source: slidetodoc.com
Source: slidetodoc.com
Calculate the mass of a 2 moles and b 025 moles of iron. Mass number of moles molar mass. Moles mass molar mass nmM. The molar mass is simply the mass of one mole of substance ie. N 5988 g 18015 gmol 3324 mol.
 Source: slideplayer.com
Source: slideplayer.com
Convert grams Glycine to moles or moles Glycine to grams. As you already know how the grams to moles conversion work find the number of moles. How do you convert moles to liters per molarity. The mass of the sample containing about 6 023 1 0 23 6023 times 10 23 6 023 1 0 23 atoms or molecules see Avogadro number. At 0C 32F or 27315K at standard atmospheric pressureDefinitions of molecular mass molecular weight molar mass and.
 Source: pinterest.com
Source: pinterest.com
In each case the number of grams in 1 mol is the same as the number of atomic mass units that describe the atomic mass the molecular mass or the formula mass respectively. This idea is very critical in chemistry because it is used all the time. 2 A compound with an empirical formula of C4H4O and a molar mass of 136 grams per mole. For a molecule with a weight of 18 the molar mass is 18 grams. It is the amount of grams that is numerically equal to the molecular weight.
 Source: slidetodoc.com
Source: slidetodoc.com
21008 11599 18006 which when rounded for sig figs is 1801 gmol. At 0C 32F or 27315K at standard atmospheric pressureDefinitions of molecular mass molecular weight molar mass and. Mass and mole conversions The mass and molar quantities of a substance can be easily interconverted by using the molecular weight as a conversion factor. Convert grams Glycine to moles or moles Glycine to grams. 12010714 10079418 1400672 1599945 Percent composition by element.
 Source: slidetodoc.com
Source: slidetodoc.com
To calculate the number of moles in a solution given the molarity we multiply the molarity by total volume of the solution in liters. For a molecule with a weight of 32 th. Since the unified atomic mass unit symbol. The mass of the sample containing about 6 023 1 0 23 6023 times 10 23 6 023 1 0 23 atoms or molecules see Avogadro number. Hydrogen- 51 grams 1 mol 101 g 505 moles.
 Source: slidetodoc.com
Source: slidetodoc.com
1007942 159994 Percent composition by element. This compound is also known as Water or Dihydrogen Monoxide. You can always use our grams to moles calculator to check the result. U or Da is defined as 112 of the mass of the 12 C atom it follows that the molar mass of a substance measured in grams per mole is numerically equal to its mean atomic or molecular mass measured in Da. 21008 11599 18006 which when rounded for sig figs is 1801 gmol.
 Source: slidetodoc.com
Source: slidetodoc.com
Substitute the values into the equation and solve for mass g. 22989773 30973761 1599944 Percent composition by element. 1007942 159994 Percent composition by element. The mass of the sample containing about 6 023 1 0 23 6023 times 10 23 6 023 1 0 23 atoms or molecules see Avogadro number. 140067 1007942 120107 1007942 120107 159994 159994 100794 Percent composition by element.
 Source: brainly.com
Source: brainly.com
Therefore the mole is a unit for that physical quantity. The unit of molar mass in the SI system is kilogram per mole. A molar mass is the weight in grams of one mole. 1 A compound with an empirical formula of C2OH4 and a molar mass of 88 grams per mole. Molar mass of NH2CH2COOH 750666 gmol.
 Source: slideplayer.com
Source: slideplayer.com
To calculate the number of moles in a solution given the molarity we multiply the molarity by total volume of the solution in liters. 140067 1007942 120107 1007942 120107 159994 159994 100794 Percent composition by element. When we weigh one mole of a substance on a balance this is called a molar mass and has the units gmol grams per mole. Mass and mole conversions The mass and molar quantities of a substance can be easily interconverted by using the molecular weight as a conversion factor. This compound is also known as Water or Dihydrogen Monoxide.
 Source: slideplayer.com
Source: slideplayer.com
Convert grams Na3PO4 to moles or moles Na3PO4 to grams. Carbon- 495 grams 1 mol 120 g 413 moles. Mass moles molar mass or m n M Step 4. 3 A compound with an empirical formula of CFBrO and a molar mass of 2547 grams per mole. Substitute the values into the equation and solve for mass g.
 Source: pinterest.com
Source: pinterest.com
Which of the following procedures should you carry out to make a 1 M solution of glucose. N 5988 g 18015 gmol 3324 mol. Convert grams H2O to moles or moles H2O to grams. One mole contains 6022 x 10 23 entities. Which of the following procedures should you carry out to make a 1 M solution of glucose.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title molar mass of 18 grams per mole by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






