Your Mol of oxygen equals how many grams images are available in this site. Mol of oxygen equals how many grams are a topic that is being searched for and liked by netizens now. You can Get the Mol of oxygen equals how many grams files here. Get all royalty-free images.
If you’re searching for mol of oxygen equals how many grams images information related to the mol of oxygen equals how many grams interest, you have pay a visit to the ideal site. Our site always gives you hints for refferencing the maximum quality video and picture content, please kindly hunt and find more informative video articles and images that fit your interests.
Mol Of Oxygen Equals How Many Grams. Thus 4 moles of oxygen gas O2 would have a mass of 128 g. We assume you are converting between grams Oxygen and mole. Likewise how many grams is 1667 moles of oxygen. Density of oxygen is equal to 1429 kgm³.
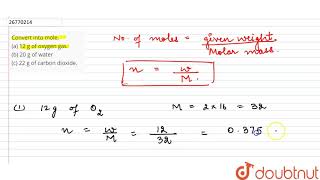 Convert Into Mole A 12 G Of Oxygen Gas B 20 G Of Water C 22 G Of Carbon Dioxide Youtube From youtube.com
Convert Into Mole A 12 G Of Oxygen Gas B 20 G Of Water C 22 G Of Carbon Dioxide Youtube From youtube.com
Since 1 mole of oxygen is equivalent to 32 g 4 moles of oxygen gas would be equivalent to 4 moles x 32 gmole 128 g. The molecular formula for Oxygen is O. Molecular weight of Oxygen or mol The molecular formula for Oxygen is O. Molecular weight of Oxygen or grams. One mole equals 6 x 1023 atomsmoleculeswhatever. 1 cubic meter of Oxygen weighs 1429 kilograms kg 1 cubic inch of Oxygen weighs 0000826014 ounce oz Oxygen weighs 0001429 gram per cubic centimeter or 1429 kilogram per cubic meter ie.
Thus 4 moles of oxygen gas O2 would have a mass of 128 g.
How many moles of oxygen are in 1 mole. The answer is 159994. One mole of Water is composed of 1 mole of Oxygen and two moles of Hydrogen. These definitions allow a new definition of atomic weight. Oxygen weighs 0001429 gram per cubic centimeter or 1429 kilogram per cubic meter ie. The answer is 159994.
 Source: youtube.com
Source: youtube.com
Since oxygen has an atomic mass of 16 gmole the molar mass of oxygen gas O2 is 2 x 16 gmole 32 gmole. The mass of oxygen equal to one mole of oxygen is 15998 grams and the mass of one mole of hydrogen is 1008 g. You can view more details on each measurement unit. The mole and the amu are related. These definitions allow a new definition of atomic weight.
 Source: youtube.com
Source: youtube.com
You can view more details on each measurement unit. Besides how many moles are in 16 grams of oxygen. At 0C 32F or 27315K at standard atmospheric pressure. 1 mole elemental oxygen16 grams O1 mole 16 grams. So take the molecular mass of elemental oxygen - 16 grams per mole.
 Source: youtube.com
Source: youtube.com
One mole equals 6 x 1023 atomsmoleculeswhatever. Correspondingly how many moles are in 16 grams of oxygen. Here we are given with 16 g of oxygen. Thus the number of molecules in 16 gm or 05 mole of oxygen gas are 05 x 6023 x 1023 3012 x 1023 molecules. At 0C 32F or 27315K at standard atmospheric pressure.

Then convert from moles to grams. So the answer would be 23584-22347grams divided. The mass of oxygen equal to one mole of oxygen is 15998 grams and the mass of one mole of hydrogen is 1008 g. The answer is 159994. 16 grams or 32 grams depending on what youre looking atThe mass of one mole of oxygen atoms is about 16 grams but oxygen gas exists as a molecule O2 so 1 mole of.
 Source: youtube.com
Source: youtube.com
The molar mass is the mass in grams when this number of molecules is equal to Avogadros number one mole. The SI base unit for amount of substance is the mole. Molecular weight of Oxygen or mol The molecular formula for Oxygen is O. Molar mass of oxygen 162gm 32gm. The mass of oxygen equal to one mole of oxygen is 15998 grams and the mass of one mole of hydrogen is 1008 g.
 Source: pinterest.com
Source: pinterest.com
Here we are given with 16 g of oxygen. Molecular weight of Oxygen or mol The molecular formula for Oxygen is O. In other words 1 mole of oxygen would contain molecules. 1 mole elemental oxygen16 grams O1 mole 16 grams. The mass of oxygen equal to one mole of oxygen is 15998 grams and the mass of one mole of hydrogen is 1008 g.
 Source: pinterest.com
Source: pinterest.com
Note that rounding errors may occur so always check the results. You can view more details on each measurement unit. So the answer would be 23584-22347grams divided. The molecular formula for Oxygen is O. How many grams of O one atom oxygen 123 X 1024 molecules H2O 1 mole H2O6022 X 10231 mole O1 mole H2O16 grams1 mole O 327 grams of oxygen.
 Source: pinterest.com
Source: pinterest.com
Molecular weight of Oxygen or mol The molecular formula for Oxygen is O. 1 cubic meter of Oxygen weighs 1429 kilograms kg 1 cubic inch of Oxygen weighs 0000826014 ounce oz Oxygen weighs 0001429 gram per cubic centimeter or 1429 kilogram per cubic meter ie. Thus 4 moles of oxygen gas O2 would have a mass of 128 g. These definitions allow a new definition of atomic weight. You can view more details on each measurement unit.
 Source: youtube.com
Source: youtube.com
You must mean. One mole of Water is composed of 1 mole of Oxygen and two moles of Hydrogen. We have to find the moles of oxygen. How many moles of oxygen are in 1 mole. 1 mole elemental oxygen16 grams O1 mole 16 grams.

We assume you are converting between grams Oxygen and mole. Correspondingly how many moles are in 16 grams of oxygen. Since oxygen has an atomic mass of 16 gmole the molar mass of oxygen gas O2 is 2 x 16 gmole 32 gmole. Thus mass of 1 mole of oxygen is 32gm. 1 cubic meter of Oxygen weighs 1429 kilograms kg 1 cubic inch of Oxygen weighs 0000826014 ounce oz Oxygen weighs 0001429 gram per cubic centimeter or 1429 kilogram per cubic meter ie.
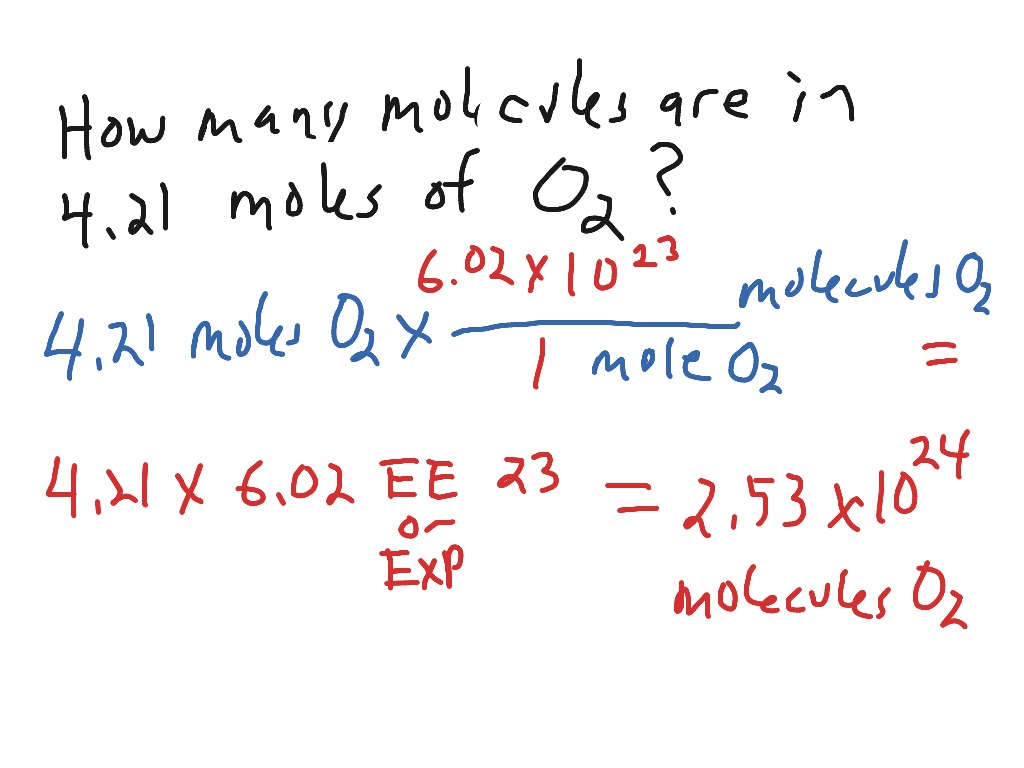 Source: showme.com
Source: showme.com
Use this page to learn how to convert between moles Oxygen and gram. How many moles of oxygen are in 1 mole. 16 grams or 32 grams depending on what youre looking atThe mass of one mole of oxygen atoms is about 16 grams but oxygen gas exists as a molecule O2 so 1 mole of. The mole and the amu are related. So take the molecular mass of elemental oxygen - 16 grams per mole.
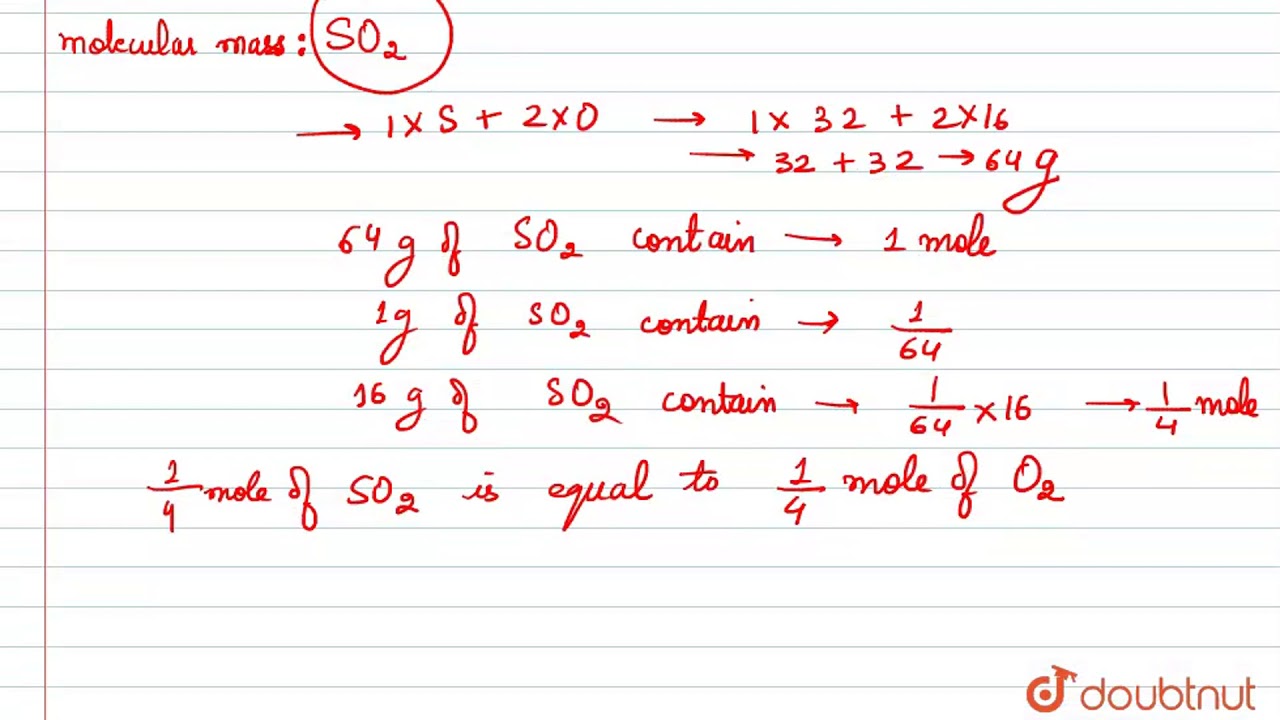 Source: m.youtube.com
Source: m.youtube.com
The atomic mass of oxygen is 1600 amu. One mole of Water is composed of 1 mole of Oxygen and two moles of Hydrogen. Molecular weight of Oxygen or mol The molecular formula for Oxygen is O. The SI base unit for amount of substance is the mole. The number of molecules in 16 gm of oxygen are 05 1632 moles.
 Source: pinterest.com
Source: pinterest.com
In other words 1 mole of oxygen would contain molecules. The molar mass is the mass in grams when this number of molecules is equal to Avogadros number one mole. You can view more details on each measurement unit. The mole and the amu are related. 1 grams Oxygen is equal to 0062502343837894 mole.
 Source: youtube.com
Source: youtube.com
We assume you are converting between grams Oxygen and mole. 16 grams or 32 grams depending on what youre looking atThe mass of one mole of oxygen atoms is about 16 grams but oxygen gas exists as a molecule O2 so 1 mole of. The number of molecules in 16 gm of oxygen are 05 1632 moles. Mass of P2O5 P 2 O 5 is 0290 g. Likewise people ask how many moles are in 16 grams of oxygen.
 Source: youtube.com
Source: youtube.com
The number of molecules in 16 gm of oxygen are 05 1632 moles. How many grams Oxygen in 1 mol. One mole of Water is composed of 1 mole of Oxygen and two moles of Hydrogen. Thus the number of molecules in 16 gm or 05 mole of oxygen gas are 05 x 6023 x 1023 3012 x 1023 molecules. One mole equals 6 x 1023 atomsmoleculeswhatever.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title mol of oxygen equals how many grams by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






