Your Mass of one mole oxygen grams images are available. Mass of one mole oxygen grams are a topic that is being searched for and liked by netizens now. You can Get the Mass of one mole oxygen grams files here. Download all free photos.
If you’re looking for mass of one mole oxygen grams pictures information linked to the mass of one mole oxygen grams keyword, you have visit the right site. Our website always provides you with hints for downloading the highest quality video and image content, please kindly hunt and locate more informative video articles and graphics that match your interests.
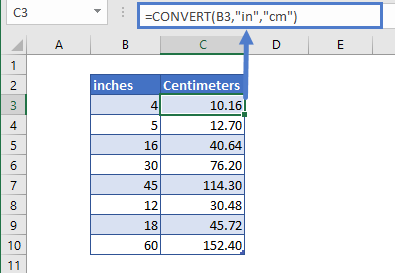
Mass Of One Mole Oxygen Grams. There are 12 things in a dozen and 602 hexillion things in a mole. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. The M r of sodium oxide is 23 2 16 62. The relative formula mass of a substance shown in grams is called one mole of that.
 Find The Mass Of 1 Molecule Of Oxygen In Amu U And In Gram Brainly In From brainly.in
Find The Mass Of 1 Molecule Of Oxygen In Amu U And In Gram Brainly In From brainly.in
What is grams per mole equal to. The number 6022140761023 the Avogadro number was chosen so that the mass of one mole of a chemical compound in grams is numerically equal for most practical purposes to the average mass of one molecule of the compound in daltons. The A r of sodium is 23 and the A r of oxygen is 16. The M r of sodium oxide is 23 2 16 62. When a 1360-g sample of a compound containing only magnesium and oxygen is decomposed 540 g. It is a name for a specific number of things.
Finding molar mass starts with units of grams per mole gmol.
Finding molar mass starts with units of grams per mole gmol. The relative formula mass of a substance shown in grams is called one mole of that. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. The number 6022140761023 the Avogadro number was chosen so that the mass of one mole of a chemical compound in grams is numerically equal for most practical purposes to the average mass of one molecule of the compound in daltons. What is grams per mole equal to.
 Source: slideplayer.com
Source: slideplayer.com
Well talk about wh. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. What is grams per mole equal to. Finding molar mass starts with units of grams per mole gmol. A mole is like a dozen.
 Source: youtube.com
Source: youtube.com
When a 1360-g sample of a compound containing only magnesium and oxygen is decomposed 540 g. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. The M r of sodium oxide is 23 2 16 62. Finding molar mass starts with units of grams per mole gmol. The A r of sodium is 23 and the A r of oxygen is 16.
 Source: toppr.com
Source: toppr.com
The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. Finding molar mass starts with units of grams per mole gmol. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. The mass of water is 1800 grams per mole.

What is grams per mole equal to. There are 12 things in a dozen and 602 hexillion things in a mole. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. What is the of oxygen in water. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance.
 Source: youtube.com
Source: youtube.com
The A r of sodium is 23 and the A r of oxygen is 16. What is grams per mole equal to. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. There are 12 things in a dozen and 602 hexillion things in a mole. The A r of sodium is 23 and the A r of oxygen is 16.
 Source: youtube.com
Source: youtube.com
When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. Finding molar mass starts with units of grams per mole gmol. Well talk about wh. What is the of oxygen in water.
 Source: brainly.in
Source: brainly.in
The number 6022140761023 the Avogadro number was chosen so that the mass of one mole of a chemical compound in grams is numerically equal for most practical purposes to the average mass of one molecule of the compound in daltons. The relative formula mass of a substance shown in grams is called one mole of that. Finding molar mass starts with units of grams per mole gmol. There are 12 things in a dozen and 602 hexillion things in a mole. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
 Source: youtube.com
Source: youtube.com
The relative formula mass of a substance shown in grams is called one mole of that. When a 1360-g sample of a compound containing only magnesium and oxygen is decomposed 540 g. The A r of sodium is 23 and the A r of oxygen is 16. What is grams per mole equal to. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance.

When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. The relative formula mass of a substance shown in grams is called one mole of that. Finding molar mass starts with units of grams per mole gmol. What is the of oxygen in water. There are 12 things in a dozen and 602 hexillion things in a mole.
 Source: youtube.com
Source: youtube.com
What is grams per mole equal to. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. There are 12 things in a dozen and 602 hexillion things in a mole. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. Well talk about wh.
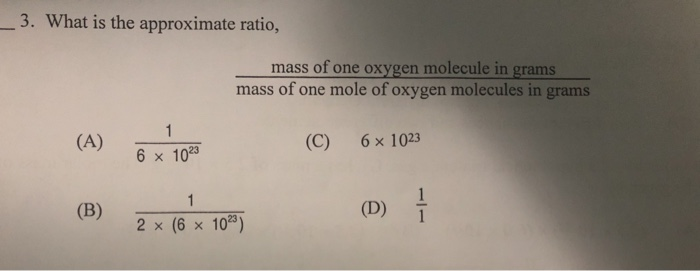 Source: chegg.com
Source: chegg.com
Finding molar mass starts with units of grams per mole gmol. The mass of water is 1800 grams per mole. Finding molar mass starts with units of grams per mole gmol. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. Finding molar mass starts with units of grams per mole gmol.
 Source: slideplayer.com
Source: slideplayer.com
What is grams per mole equal to. The mass of water is 1800 grams per mole. Well talk about wh. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. When a 1360-g sample of a compound containing only magnesium and oxygen is decomposed 540 g.

When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. What is the of oxygen in water. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. The A r of sodium is 23 and the A r of oxygen is 16.
 Source: youtube.com
Source: youtube.com
Finding molar mass starts with units of grams per mole gmol. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. What is the of oxygen in water. The M r of sodium oxide is 23 2 16 62. What is grams per mole equal to.
 Source: youtube.com
Source: youtube.com
The mass of water is 1800 grams per mole. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. Finding molar mass starts with units of grams per mole gmol. The number 6022140761023 the Avogadro number was chosen so that the mass of one mole of a chemical compound in grams is numerically equal for most practical purposes to the average mass of one molecule of the compound in daltons. The A r of sodium is 23 and the A r of oxygen is 16.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title mass of one mole oxygen grams by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.