Your Mass in grams of one mole of water images are available in this site. Mass in grams of one mole of water are a topic that is being searched for and liked by netizens now. You can Download the Mass in grams of one mole of water files here. Get all royalty-free images.
If you’re looking for mass in grams of one mole of water pictures information connected with to the mass in grams of one mole of water keyword, you have come to the right site. Our website frequently gives you suggestions for seeking the highest quality video and picture content, please kindly hunt and find more informative video articles and images that match your interests.
Mass In Grams Of One Mole Of Water. Mass percent is also known as percent by weight or ww. Water has 3 atoms two hydrogen atoms and one oxygen atom. The mass in grams of 1 mole of substance is its molar mass. It is a name for a specific number of things.
 Mole Fraction Easy Science Mole Fraction Easy Science Fractions From pinterest.com
Mole Fraction Easy Science Mole Fraction Easy Science Fractions From pinterest.com
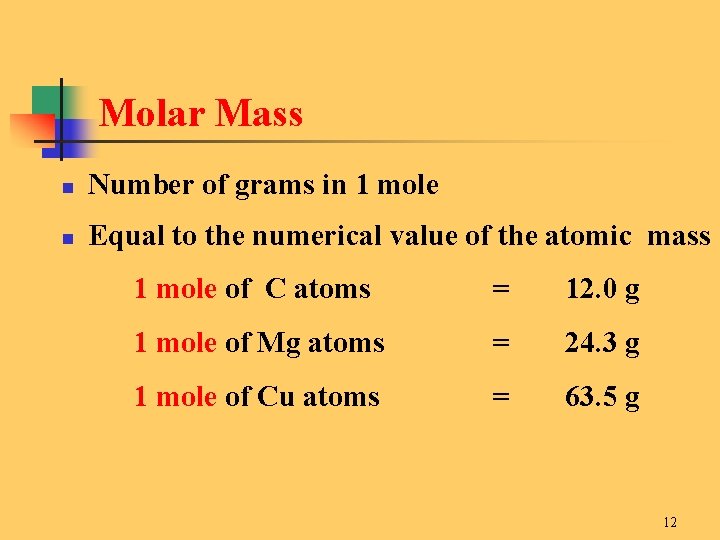
For example the average mass of one molecule of water is about 180153 daltons and one mole of water is about 180153 grams. The answer has three significant figures because the given mass has three significant figures. The molar mass is the sum of the masses of all the atoms in one mole of the compound. The units of mass are typically grams. The molar mass of KMnO 4 is 158034 gramsmole. Gram Atomic Mass and Gram Molecular Mass.
How many grams of hydrogen gas are needed to produce 1050 grams of water given the following unbalanced chemical reaction.
The mass of tin is less than one mole but the 12 ratio means that more than one mole of ceHF is required for the reaction. What is the of oxygen in water. Also the atomic weight of hydrogen is 1 gram and oxygen is 16 grams. 18 grams per mole is the molar mass of water. For one mole the atomic or molecular mass will be the same as the weight. 1 Balance the chemical equation.
 Source: pinterest.com
Source: pinterest.com
N2g 3 H 2g 2 NH 3g How many grams of NH 3 would. 1 moles Water to grams 1801528 grams. Molar mass of a Substance Mass of the Substance in gramsNumber of Moles For example the molar mass of water is approximately 18015 gmol which is the mass of N A number of water molecules. How many grams of hydrogen gas are needed to produce 1050 grams of water given the following unbalanced chemical reaction. 18 grams per mole is the molar mass of water.
 Source: slideplayer.com
Source: slideplayer.com
For one mole the atomic or molecular mass will be the same as the weight. 2 Convert grams of the substance given. A protein whose molecule has an average mass of 64 kDa would have a molar mass of 64 kgmol. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. Its easier to grasp how much water is in a mole if you find the volume of this amount of mass.
 Source: pinterest.com
Source: pinterest.com
N2g 3 H 2g 2 NH 3g How many grams of NH 3 would. Its easier to grasp how much water is in a mole if you find the volume of this amount of mass. N2g 3 H 2g 2 NH 3g How many grams of NH 3 would. A mole is like a dozen. Use the molar mass to convert from moles to grams The number of grams of a substance per mole Mass g Compound A Moles Compound A Moles Compound B Mass g Compound B Molar mass Molar mass Molar X ratio Moles and Chemical Reactions Chapter 4 Stoichiometry For the following reaction.
 Source: pinterest.com
Source: pinterest.com
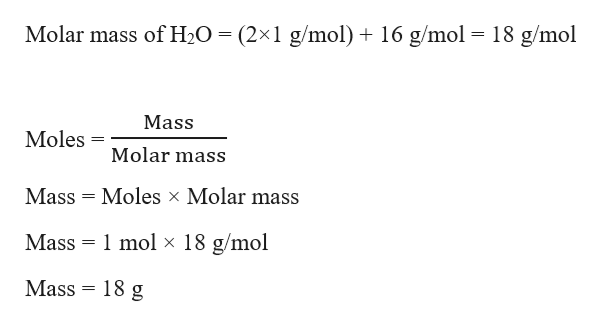
It is a name for a specific number of things. 2 Convert grams of the substance given. Unless you have a good sense of mass this value probably doesnt have much meaning to you. The gram atomic mass of an element is the mass of one mole of that element. We calculate the molar mass of one water molecule when we add 2 x 1 16 18 grams.
 Source: pinterest.com
Source: pinterest.com
Water has 3 atoms two hydrogen atoms and one oxygen atom. The units of mass are typically grams. I rounded off some but I made sure to keep more digits. What is the of oxygen in water. The sum of all the mass percentages should add up to 100.
 Source: bartleby.com
Source: bartleby.com
When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. The sum of all the mass percentages should add up to 100. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. Molar mass of a Substance Mass of the Substance in gramsNumber of Moles For example the molar mass of water is approximately 18015 gmol which is the mass of N A number of water molecules. 1 Balance the chemical equation.
 Source: pinterest.com
Source: pinterest.com
How many grams of hydrogen gas are needed to produce 1050 grams of water given the following unbalanced chemical reaction. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. When a 1360-g sample of a compound containing only magnesium and oxygen is decomposed 540 g. I rounded off some but I made sure to keep more digits. Water has 3 atoms two hydrogen atoms and one oxygen atom.
 Source: slidetodoc.com
Source: slidetodoc.com
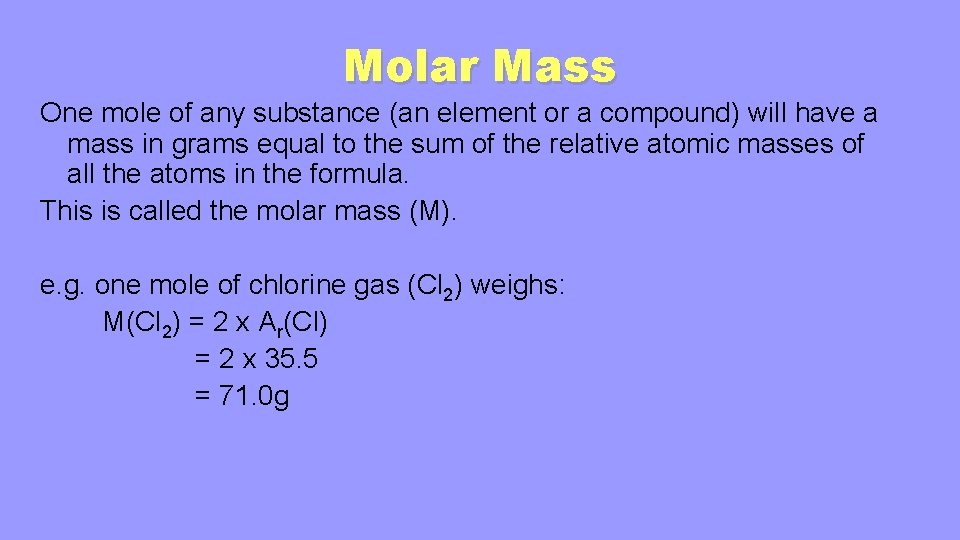
Due to the use of the same reference substance in defining the atomic mass unit and the mole the formula mass amu and molar mass gmol for any substance are numerically equivalent for example one H 2 O molecule weighs approximately18 amu and 1 mole of H 2 O molecules weighs. Finding molar mass starts with units of grams per mole gmol. Divide the grams given in the problem by the substances molar mass. The mass in grams of 1 mole of substance is its molar mass. N2g 3 H 2g 2 NH 3g How many grams of NH 3 would.
 Source: pinterest.com
Source: pinterest.com
The sum of all the mass percentages should add up to 100. 1050 g 18015 gmol 582848 mol of H 2 O. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. For one mole the atomic or molecular mass will be the same as the weight. Use the molar mass to convert from moles to grams The number of grams of a substance per mole Mass g Compound A Moles Compound A Moles Compound B Mass g Compound B Molar mass Molar mass Molar X ratio Moles and Chemical Reactions Chapter 4 Stoichiometry For the following reaction.
 Source: pinterest.com
Source: pinterest.com
The amount of grams in a mole depends on the substance you have. The mass of tin is less than one mole but the 12 ratio means that more than one mole of ceHF is required for the reaction. Divide the grams given in the problem by the substances molar mass. Watch for rounding errors in the last significant figure to make sure all the percentages add up. Well talk about wh.
 Source: pinterest.com
Source: pinterest.com
Finding molar mass starts with units of grams per mole gmol. When a 1360-g sample of a compound containing only magnesium and oxygen is decomposed 540 g. 18 grams per mole is the molar mass of water. 2H 2 O 2— 2H 2 O. 1 moles Water to grams 1801528 grams.
 Source: youtube.com
Source: youtube.com
The sum of all the mass percentages should add up to 100. In addition the. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. What is the of oxygen in water. Therefore one mole of water weighs 180152 grams.
 Source: slidetodoc.com
Source: slidetodoc.com
2 Convert grams of the substance given. 1 moles Water to grams 1801528 grams. Mass percent is also known as percent by weight or ww. Finding molar mass starts with units of grams per mole gmol. Finding molar mass starts with units of grams per mole gmol.
 Source: youtube.com
Source: youtube.com
Also the atomic weight of hydrogen is 1 gram and oxygen is 16 grams. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. A protein whose molecule has an average mass of 64 kDa would have a molar mass of 64 kgmol. For one mole the atomic or molecular mass will be the same as the weight. For example the average mass of one molecule of water is about 180153 daltons and one mole of water is about 180153 grams.
 Source: slideplayer.com
Source: slideplayer.com
Unless you have a good sense of mass this value probably doesnt have much meaning to you. The amount of grams in a mole depends on the substance you have. To work it out find the atomic or molecular mass of your substance and multiply it by the number of moles you have. The weight of one-half mole of water is 18015 x 05 90075 g. Use the molar mass to convert from moles to grams The number of grams of a substance per mole Mass g Compound A Moles Compound A Moles Compound B Mass g Compound B Molar mass Molar mass Molar X ratio Moles and Chemical Reactions Chapter 4 Stoichiometry For the following reaction.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title mass in grams of one mole of water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.