Your Mass in grams of 1 mole of carbon dioxide images are ready. Mass in grams of 1 mole of carbon dioxide are a topic that is being searched for and liked by netizens now. You can Get the Mass in grams of 1 mole of carbon dioxide files here. Find and Download all free photos.
If you’re looking for mass in grams of 1 mole of carbon dioxide images information linked to the mass in grams of 1 mole of carbon dioxide interest, you have come to the ideal site. Our site frequently provides you with hints for seeing the maximum quality video and picture content, please kindly hunt and find more informative video content and images that match your interests.
Mass In Grams Of 1 Mole Of Carbon Dioxide. Because the unknown gas is from a fire extinguisher we suspect that it is carbon dioxide. Therefore the molar mass of H 2 O is 1801528. The mass of one mole of a substance measured in grams. The atomic mass of hydrogen is 100794 and oxygen is 159994.
 Pin On Chemistry From pinterest.com
Pin On Chemistry From pinterest.com
The atomic mass of atoms of all other elements is determined relative to the mass of carbon-12. Therefore the molar mass of H 2 O is 1801528. Under standard temperature pressure conditions what is the volume of 1 mol of gas. This is known as the molar mass M and has the units g mol-1 gmol grams per mole of substance The relationship between molar mass mass and moles can be expressed as a mathematical equation as shown below. The number of units in one mole. Therefore 1 mole of CO 2 contains 1201 grams of carbon and 32 grams of oxygen.
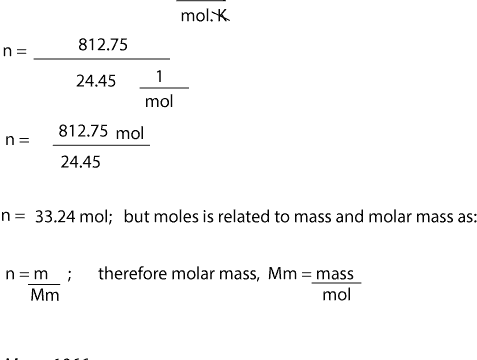
How many mol of CO₂ carbon dioxide are present in 555 L.
This is known as the molar mass M and has the units g mol-1 gmol grams per mole of substance The relationship between molar mass mass and moles can be expressed as a mathematical equation as shown below. Therefore 1 mole of CO 2 contains 1201 grams of carbon and 32 grams of oxygen. 1 mole of a pure substance has a mass equal to its molecular mass 1 expressed in grams. The mass of one mole of a substance measured in grams. Molecular mass of SiO₂ 601u 1 mole of SiO₂ 601g 08 mol of SiO₂ 601 08 481g Q11. By summing the molar mass for CO 2 4401 gmol we help confirm that the unknown gas is carbon dioxide.
 Source: pinterest.com
Source: pinterest.com
The number of units in one mole. Therefore 1 mole of CO 2 contains 1201 grams of carbon and 32 grams of oxygen. The atomic mass of atoms of all other elements is determined relative to the mass of carbon-12. Because the unknown gas is from a fire extinguisher we suspect that it is carbon dioxide. By summing the molar mass for CO 2 4401 gmol we help confirm that the unknown gas is carbon dioxide.
 Source: pinterest.com
Source: pinterest.com
Molar mass 100794 100794 159994. 1 mole of CO 2 contains 1 mole of carbon and 2 moles of oxygen. The mass of one carbon-12 atom is set at 12 amu. The mass of one mole of a substance measured in grams. Because the unknown gas is from a fire extinguisher we suspect that it is carbon dioxide.
 Source: pinterest.com
Source: pinterest.com
Since there are two hydrogen atoms per molecule and one oxygen atom the formula should look like this. The number of units in one mole. Since there are two hydrogen atoms per molecule and one oxygen atom the formula should look like this. 224 litres or 224l Q12. 1 mole of CO 2 contains 1 mole of carbon and 2 moles of oxygen.
 Source: pinterest.com
Source: pinterest.com
How many grams of carbon can be found in 1 mole of carbon dioxide. The mass of one carbon-12 atom is set at 12 amu. The molar mass of carbon is 120107 gmol. Molecular mass of SiO₂ 601u Answer. 1 mole of CO 2 contains 1 mole of carbon and 2 moles of oxygen.
 Source: gr.pinterest.com
Source: gr.pinterest.com
The mass of one mole of a substance measured in grams. Molar mass 100794 100794 159994. 224 litres or 224l Q12. 1 mole of CO 2 contains 1 mole of carbon and 2 moles of oxygen. 6022 1023 which is the number of atoms in 12 grams of carbon-12.
 Source: in.pinterest.com
Source: in.pinterest.com
Molecular mass of SiO₂ 601u Answer. Molar mass 100794 100794 159994. 6022 1023 which is the number of atoms in 12 grams of carbon-12. How many grams of carbon can be found in 1 mole of carbon dioxide. Because the unknown gas is from a fire extinguisher we suspect that it is carbon dioxide.
 Source: pinterest.com
Source: pinterest.com
1 mole of CO 2 contains 1 mole of carbon and 2 moles of oxygen. Molar mass 100794 100794 159994. The atomic mass of hydrogen is 100794 and oxygen is 159994. Molecular mass of SiO₂ 601u 1 mole of SiO₂ 601g 08 mol of SiO₂ 601 08 481g Q11. 1 mole of a pure substance has a mass equal to its molecular mass 1 expressed in grams.
 Source: gr.pinterest.com
Source: gr.pinterest.com
How many grams of carbon can be found in 1 mole of carbon dioxide. 6022 1023 which is the number of atoms in 12 grams of carbon-12. 1 mole of CO 2 contains 1 mole of carbon and 2 moles of oxygen. Example Exercise 99 Molar Mass of a Gas We apply the unit factor 1 mol224 L to cancel liters which appears in the denominator. Since there are two hydrogen atoms per molecule and one oxygen atom the formula should look like this.
 Source: in.pinterest.com
Source: in.pinterest.com
The atomic mass of hydrogen is 100794 and oxygen is 159994. The atomic mass of atoms of all other elements is determined relative to the mass of carbon-12. Since there are two hydrogen atoms per molecule and one oxygen atom the formula should look like this. Molecular mass of SiO₂ 601u Answer. Under standard temperature pressure conditions what is the volume of 1 mol of gas.
 Source: pinterest.com
Source: pinterest.com
Since there are two hydrogen atoms per molecule and one oxygen atom the formula should look like this. How many grams of carbon can be found in 1 mole of carbon dioxide. The atomic mass of atoms of all other elements is determined relative to the mass of carbon-12. Since there are two hydrogen atoms per molecule and one oxygen atom the formula should look like this. The mass of one carbon-12 atom is set at 12 amu.
 Source: gr.pinterest.com
Source: gr.pinterest.com
1 mole of CO 2 contains 1 mole of carbon and 2 moles of oxygen. The mass of one mole of a substance measured in grams. This is known as the molar mass M and has the units g mol-1 gmol grams per mole of substance The relationship between molar mass mass and moles can be expressed as a mathematical equation as shown below. The atomic mass of hydrogen is 100794 and oxygen is 159994. Since there are two hydrogen atoms per molecule and one oxygen atom the formula should look like this.
 Source: pinterest.com
Source: pinterest.com
1 mole of CO 2 contains 1 mole of carbon and 2 moles of oxygen. How many mol of CO₂ carbon dioxide are present in 555 L. 1 mole of CO 2 contains 1 mole of carbon and 2 moles of oxygen. Example Exercise 99 Molar Mass of a Gas We apply the unit factor 1 mol224 L to cancel liters which appears in the denominator. Molecular mass of SiO₂ 601u 1 mole of SiO₂ 601g 08 mol of SiO₂ 601 08 481g Q11.
 Source: pinterest.com
Source: pinterest.com
Because the unknown gas is from a fire extinguisher we suspect that it is carbon dioxide. Molecular mass of SiO₂ 601u 1 mole of SiO₂ 601g 08 mol of SiO₂ 601 08 481g Q11. Molar mass 100794 100794 159994. 1 mole of a pure substance has a mass equal to its molecular mass 1 expressed in grams. 1 mole of CO 2 contains 1 mole of carbon and 2 moles of oxygen.
 Source: pinterest.com
Source: pinterest.com
The molar mass of carbon is 120107 gmol. Therefore the molar mass of H 2 O is 1801528. Molecular mass of SiO₂ 601u Answer. 6022 1023 which is the number of atoms in 12 grams of carbon-12. Therefore 1 mole of CO 2 contains 1201 grams of carbon and 32 grams of oxygen.
 Source: pinterest.com
Source: pinterest.com
1 mole of a pure substance has a mass equal to its molecular mass 1 expressed in grams. Under standard temperature pressure conditions what is the volume of 1 mol of gas. Molecular mass of SiO₂ 601u Answer. Therefore the molar mass of H 2 O is 1801528. The atomic mass of atoms of all other elements is determined relative to the mass of carbon-12.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title mass in grams of 1 mole of carbon dioxide by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.