Your Is one mole equal to one gram images are ready in this website. Is one mole equal to one gram are a topic that is being searched for and liked by netizens now. You can Find and Download the Is one mole equal to one gram files here. Find and Download all royalty-free images.
If you’re searching for is one mole equal to one gram pictures information related to the is one mole equal to one gram interest, you have pay a visit to the ideal site. Our site frequently provides you with hints for seeing the highest quality video and picture content, please kindly search and find more enlightening video articles and images that match your interests.
Is One Mole Equal To One Gram. One mole of any molecule corresponds to Avogadros number of molecules ie 602310 23 molecules. One gram of any molecule is not equal to Avogadros number of molecules. 1 gram is equal to 00227 moles. Molecular weight of In or grams.
 Definition Of Avogadro S Number Chemistry Classroom Chemistry Study Motivation From pinterest.com
Definition Of Avogadro S Number Chemistry Classroom Chemistry Study Motivation From pinterest.com
One mole of a substance is equal to 6022 10²³ units of that substance such as atoms molecules or ions. One mole is around 600 sextillion molecules. It is defined as the molar mass of any molecule equal to Avogadros number of molecules. Mole is the SI unit used to measure how many molecules or atoms there are. Created by Sal Khan. It is defined as the number of grams of a substance ie.
Molecular weight of 10 or mol.
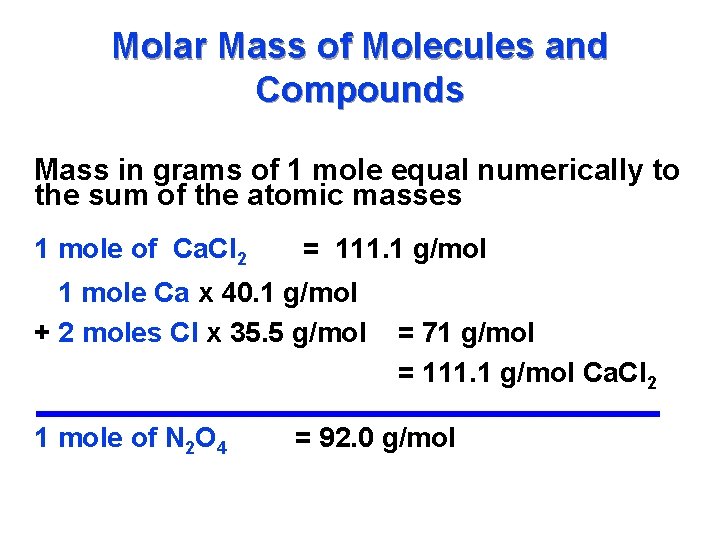
A mole is the quantity of any pure substance element or compound that contains one Avogadros number 602 1023 of molecules of that substance. Then convert from moles to grams. Molecular weight of 10 or mol. A mole is the quantity of any pure substance element or compound that contains one Avogadros number 602 1023 of molecules of that substance. The number 6022 140 76 10 23 the Avogadro number was chosen so that the mass of one mole of a chemical compound in grams is numerically equal for most practical purposes to the average mass of one molecule of the compound in daltons roughly equivalent to the number of nucleons protons or neutrons in the molecule. 30 moles 1 to grams 30 grams.
 Source: pinterest.com
Source: pinterest.com
Note that rounding errors may occur so always check the results. First of all we will define the terms like one gram atom one gram molecule and AMU units. One mole of any element is equals to its gamGram AtomicMassand one mole of any molecule is also equals to that moleculesgram atomic mass. 1 grams 10 is equal to 1 mole. Mole is the SI unit used to measure how many molecules or atoms there are.
 Source: pinterest.com
Source: pinterest.com
100 moles 1 to grams 100 grams. Note that rounding errors may occur so always check the results. Type in your own numbers in the form to convert the units. 5 moles 1 to grams 5 grams. The number 6022 10²³ is known as Avogadros number or Avogadros constant.
 Source: slideplayer.com
Source: slideplayer.com
It is defined as the molar mass of any molecule equal to Avogadros number of molecules. One mole of any element is equals to its gamGram AtomicMassand one mole of any molecule is also equals to that moleculesgram atomic mass. 12 amuatom 12 gmol. So take the molecular mass of elemental oxygen - 16 grams per mole. Type in your own numbers in the form to convert the units.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
This compound is also known as Magnesium Chloride. 1 moles 1 to grams 1 grams. Created by Sal Khan. It is defined as the molar mass of any molecule equal to Avogadros number of molecules. Think of it this way a single small chicken egg weighs 3 grams a large one weighs 5 grams.
 Source: pinterest.com
Source: pinterest.com
Scientists use this number because 1 gram of hydrogen is around 1 mole of atoms. It is defined as the number of grams of a substance ie. A mole is the quantity of any pure substance element or compound that contains one Avogadros number 602 1023 of molecules of that substance. The statement There is no difference between one mole and one gram molecule is false. Like 1 dozen 1 mole refers to an exact number.
 Source: slideplayer.com
Source: slideplayer.com
30 moles 1 to grams 30 grams. Thus one mole of hydrogen has a mass of 1 gram. 1 mole of sodium is equal to 6023 10²³ number of atoms. The mass of one mole of an element equal in grams to the atomic weight. Use this page to learn how to convert between moles In and gram.
 Source: slidetodoc.com
Source: slidetodoc.com
For example one mole of a pure carbon-12 12 C sample will have a mass of exactly 12 grams and will contain 60221407610 23 N A number of 12 C atoms. Note that rounding errors may occur so always check the results. 5 moles 1 to grams 5 grams. The SI base unit for amount of substance is the mole. 100 moles 1 to grams 100 grams.
 Source: slideplayer.com
Source: slideplayer.com
Like 1 dozen 1 mole refers to an exact number. This is definitionally Avagadros number of atoms. A liter of water is 1000g of water. 1 mole elemental oxygen16 grams O1 mole 16 grams. Molar mass refers to the mass of a very specific number of moleculesatoms.
 Source: slideplayer.com
Source: slideplayer.com
Avogadros number is the conversion factor from atomic mass units AMU to grams 1 gram 602 1023 AMU. First of all we will define the terms like one gram atom one gram molecule and AMU units. Therefore we just proved that an atomic mass unit is the same thing as grams per mole. A mole is the quantity of a substance where the mass in grams equals the molecular mass so one mole of water weighs 18g. 100 moles 1 to grams 100 grams.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
The mass of one mole of an element equal in grams to the atomic weight. 1 mole of sodium is equal to 6023 10²³ number of atoms. Number of atoms present in one mole. Use this page to learn how to convert between grams 1 and mole. 1 mole is equal to 1 moles MgCl2 or 95211 grams.
 Source: slideplayer.com
Source: slideplayer.com
First of all we will define the terms like one gram atom one gram molecule and AMU units. Now we also know that the mass of a single carbon- 12 atom is exactly 12 amu as it is an isotope. 1 amuatom 1 gmol. 1 mole is equal to 1 moles Au or 19696655 grams. Lets calculate the gram molecular mass of water h2o 2.
 Source: pinterest.com
Source: pinterest.com
Note that rounding errors may occur so always check the results. And so mass of carbon-12 12 amuatom. It just so happens that at standard temperature and pressure the density of water is 1g per mL. It is equal to atomic weight in grams. 12 amuatom 12 gmol.
 Source: pinterest.com
Source: pinterest.com
Use this page to learn how to convert between grams 1 and mole. The concept of the mole can be used to convert between mass and number of particles. So take the molecular mass of elemental oxygen - 16 grams per mole. Think of it this way a single small chicken egg weighs 3 grams a large one weighs 5 grams. 20 moles 1 to grams 20 grams.

One mole of a substance is equal to 6022 10²³ units of that substance such as atoms molecules or ions. Use this page to learn how to convert between grams 10 and mole. One mole equals 6 x 1023 atomsmoleculeswhatever. 50 moles 1 to grams 50 grams. The exact value of.

Use this page to learn how to convert between moles In and gram. The concept of the mole can be used to convert between mass and number of particles. 1 mole is equal to 1 moles Au or 19696655 grams. It is equal to Avogadro number of atoms. 1 grams 10 is equal to 1 mole.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title is one mole equal to one gram by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.