Your Is molecular weight grams per mole images are ready in this website. Is molecular weight grams per mole are a topic that is being searched for and liked by netizens now. You can Download the Is molecular weight grams per mole files here. Get all free photos and vectors.
If you’re searching for is molecular weight grams per mole images information linked to the is molecular weight grams per mole interest, you have pay a visit to the ideal site. Our website frequently provides you with suggestions for seeing the maximum quality video and picture content, please kindly hunt and locate more enlightening video articles and images that match your interests.
Is Molecular Weight Grams Per Mole. Finding molar mass starts with units of grams per mole gmol. 1 Gram per mole gmol is equal 0001 Kilogram per mole kgmol use this converter. The amount of substance is the number of moles in the sample. The only difference is that gram molecular mass specifies the mass unit to be used.
 Millis Equivalents And Moles Basics 1 Mole Of Anything Atomic Formula Or Molecular Weight In Grams 1 Mole Of Na Atomic Weight 23 23 Grams Ppt Download From slideplayer.com
Millis Equivalents And Moles Basics 1 Mole Of Anything Atomic Formula Or Molecular Weight In Grams 1 Mole Of Na Atomic Weight 23 23 Grams Ppt Download From slideplayer.com
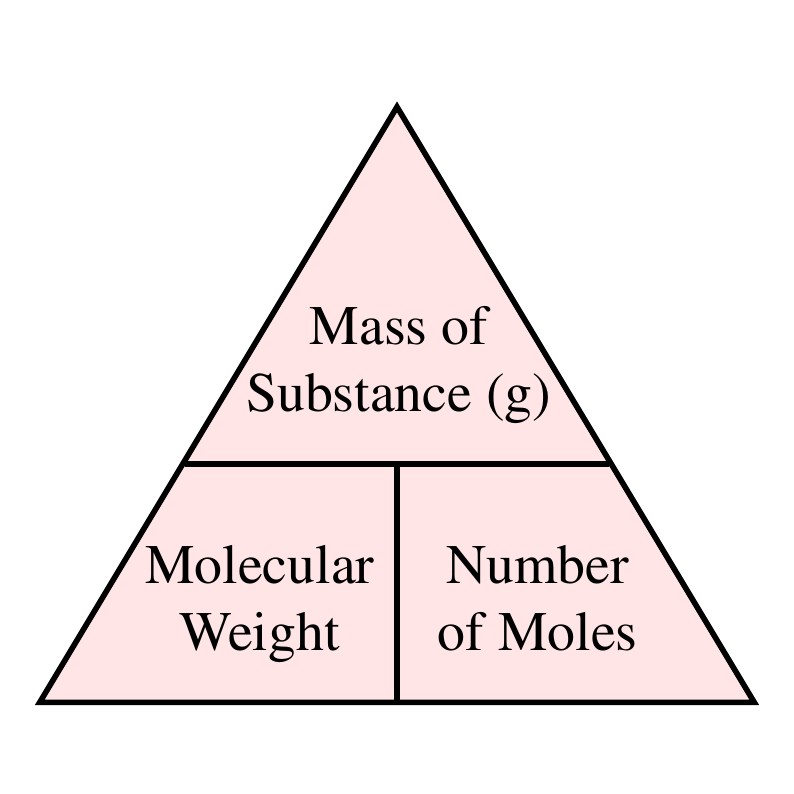
The molar massmolecular weight is actually the sum of the total mass in grams of the atoms present to make up a molecule per mole. The only difference is that gram molecular mass specifies the mass unit to be used. A common request on this site is to convert grams to moles. The unit of molar mass is gramsmole. Of gram molesVolume Mass of substance in gmMolecular massVolume. The molecular weight is the mass of one mole of a substance.
The unit of molar mass is gramsmole.
Gram per mole gmol 1. Usually the units used for this are grams per mole. There are two atoms of Mn Manganese therefore. Gram molecular mass may be reported in grams or grams per mole gmol. On the molecular scale we think in terms of molecules in moles. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance.
 Source: ncl.ac.uk
Source: ncl.ac.uk
On the other handGram per litre is mass of substance in gram dissolved in per unit volume of solution. In this movie we show how to calculate the molecular weight of a substance from the atomic weights given on the periodic table. The unit of molar mass is gramsmole. Proteins and other molecular macromolecule molecular weights are usually measured in molecular kDa or kD kilodaltons 1000 Da. A common request on this site is to convert grams to moles.
 Source: quizlet.com
Source: quizlet.com
A common request on this site is to convert grams to moles. Gram molecular mass is the mass in grams of one mole of a molecular substance. Gram molecular mass is the same as molar mass. Usually the units used for this are grams per mole. On the other handGram per litre is mass of substance in gram dissolved in per unit volume of solution.
 Source: pinterest.com
Source: pinterest.com
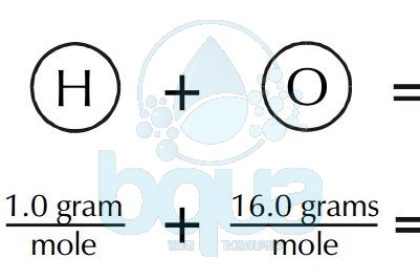
This converts atomic units to grams per mole making the molar mass of hydrogen 1007 grams per mole of carbon 120107 grams per mole of oxygen 159994 grams per mole and of chlorine 35453 grams per mole. Gram molecular mass may be reported in grams or grams per mole gmol. Gram molecular mass may be reported in grams or grams per mole gmol. Gram molecular mass is the same as molar mass. There are four atoms of O Oxygen therefore 416 64 gmol.
 Source: pinterest.com
Source: pinterest.com
Gram per mole gmol 1. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. This converts atomic units to grams per mole making the molar mass of hydrogen 1007 grams per mole of carbon 120107 grams per mole of oxygen 159994 grams per mole and of chlorine 35453 grams per mole. The relative molecular mass M r is a pure number and it does not have a unit. In this movie we show how to calculate the molecular weight of a substance from the atomic weights given on the periodic table.
 Source: pinterest.com
Source: pinterest.com
The molecular weight is the mass of one mole of a substance. Kilogram per mole kgmol 10 -3. Kilograms per mole to Grams per mole kgmol to gmol converter. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. So we can multiply the five moles needed by 75 grams per mole and we can solve that we need 375 grams of the substance.
 Source: bqua.com
Source: bqua.com
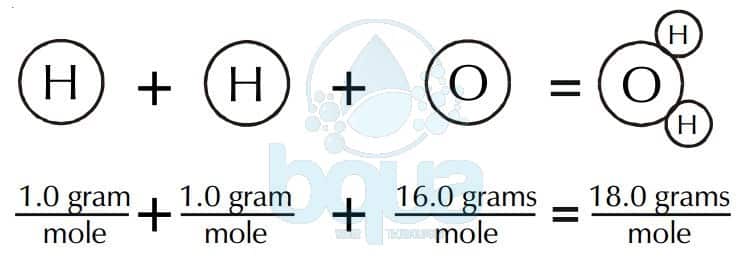
5 rows We calculate the molar mass of one water molecule when we add 2 x 1 16 18 grams. 1 g 00022 lbs. The amount of substance is the number of moles in the sample. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. The relative molecular mass M r is a pure number and it does not have a unit.
 Source: br.pinterest.com
Source: br.pinterest.com
We can write this another way using scientific notation itll be. The Molar Mass of a substance is the mass of 1 mole of that substance in multiples of the gram. In this movie we show how to calculate the molecular weight of a substance from the atomic weights given on the periodic table. A common request on this site is to convert grams to moles. The molar massmolecular weight is actually the sum of the total mass in grams of the atoms present to make up a molecule per mole.
 Source: slideplayer.com
Source: slideplayer.com
The weight of one mole is 84 grams so the weight of 7 grams is 588 grams or 0588 kilograms. Gram molecular mass is the same as molar mass. MolarityGm per moleMolecular mass. The only difference is that gram molecular mass specifies the mass unit to be used. In this movie we show how to calculate the molecular weight of a substance from the atomic weights given on the periodic table.
 Source: bqua.com
Source: bqua.com
When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. 1 gmol 22 103 lbsmol. For most practical purposes the magnitude of molar mass is numerically the same as that of the mean mass of 1 molecule expressed in Daltons. Gram molecular mass may be reported in grams or grams per mole gmol. Carbon is out there within the atomic type.
 Source: chemteam.info
Source: chemteam.info
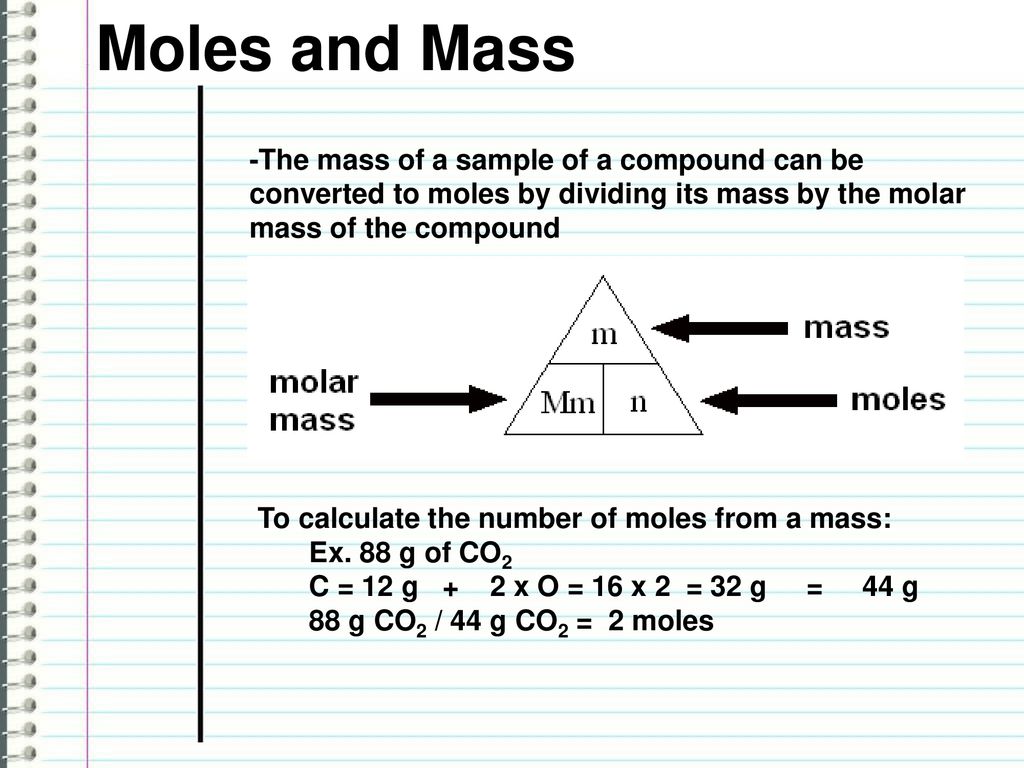
Since Gm per liter Mass of substance in gmVolume. Kilograms per mole to Grams per mole kgmol to gmol converter. A common request on this site is to convert grams to moles. Of gram molesVolume Mass of substance in gmMolecular massVolume. The molecular weight of a mole of KMn2O4 is 3910 10988 64 21298 gmol 09 Apr 2015.
 Source: pinterest.com
Source: pinterest.com
Kilograms per mole to Grams per mole kgmol to gmol converter. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. Finding molar mass starts with units of grams per mole gmol. Proteins and other molecular macromolecule molecular weights are usually measured in molecular kDa or kD kilodaltons 1000 Da. Chris Deziel holds a Bachelors degree in physics and a Masters degree in Humanities He has taught science math and English at the university level both in his native Canada and in Japan.
 Source: pinterest.com
Source: pinterest.com
The molecular weight is the mass of one mole of a substance. The weight of one mole is 84 grams so the weight of 7 grams is 588 grams or 0588 kilograms. The Molar Mass of a substance is the mass of 1 mole of that substance in multiples of the gram. Gram molecular mass is the mass in grams of one mole of a molecular substance. To complete this calculation you.
 Source: pinterest.com
Source: pinterest.com
Difference Between Formula Mass and Molecular Mass Molar mass of CCl Computing molecular weight molecular mass To calculate molecular weight of a chemical compound enter. On the other handGram per litre is mass of substance in gram dissolved in per unit volume of solution. 5 rows We calculate the molar mass of one water molecule when we add 2 x 1 16 18 grams. The relative molecular mass M r is a pure number and it does not have a unit. To complete this calculation you.
 Source: slideplayer.com
Source: slideplayer.com
When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. To complete this calculation you. Gram molecular mass may be reported in grams or grams per mole gmol. Gram molecular mass is the same as molar mass. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance.
 Source: pinterest.com
Source: pinterest.com
The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. This converts atomic units to grams per mole making the molar mass of hydrogen 1007 grams per mole of carbon 120107 grams per mole of oxygen 159994 grams per mole and of chlorine 35453 grams per mole. In this movie we show how to calculate the molecular weight of a substance from the atomic weights given on the periodic table. Molecular weight of 15756 grams per mole and also you get seven level eight eight grams. Carbon is out there within the atomic type.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title is molecular weight grams per mole by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






