Your Is grams per mol the same as amu images are available in this site. Is grams per mol the same as amu are a topic that is being searched for and liked by netizens now. You can Download the Is grams per mol the same as amu files here. Find and Download all royalty-free photos and vectors.
If you’re searching for is grams per mol the same as amu images information linked to the is grams per mol the same as amu keyword, you have come to the right site. Our site frequently provides you with hints for refferencing the highest quality video and image content, please kindly hunt and find more enlightening video content and graphics that fit your interests.
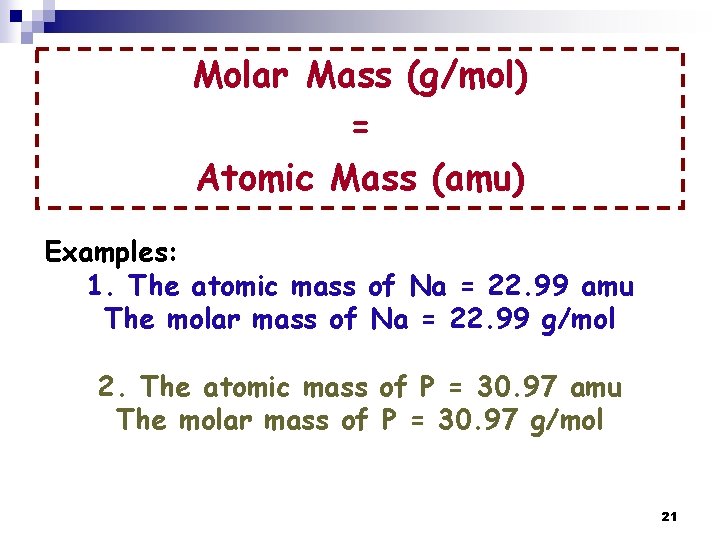
Is Grams Per Mol The Same As Amu. Although mass can be expressed as both amu and gmol gmol is the most useful system of units for laboratory chemistry. The atomic mass is useful in chemistry when it is paired with the mole concept. This number has been determined through experimentation to be 6022 1023 and is known as Avogadros number. For example carbon-12 has a mass of 12 amu.
 Moles Mole Is A Way Of Translating Between Grams And Amu S A Mole Is A Number That Helps Us Translate Between The Atomic World And Our Every Day Ppt Download From slideplayer.com
Moles Mole Is A Way Of Translating Between Grams And Amu S A Mole Is A Number That Helps Us Translate Between The Atomic World And Our Every Day Ppt Download From slideplayer.com
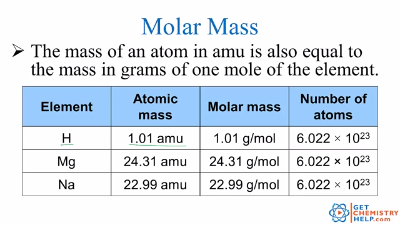
The atomic mass of an element measured in amu is the same as the mass in grams of one mole of an element. Pu1g mol-1 fracpu1 gpu6022E23 atom pu16605E-24 g atom-1 Its the same number. Pu1g mol-1 pu 1. The mass of one mole of atoms of a pure element in grams is equivalent to the atomic mass of that element in atomic mass units amu or in grams per mole gmol. The mass of an atom in amu is numerically the same as the mass of one mole of atoms of the element in grams. So mass of one nucleon 1 amu 1N1660510-24 grams.
How small is a Dalton.
The mass of a single atom of an element amu is numerically equal to the mass g of 1 mol of that element regardless of the element. 015 atomic mass units so one MOLE of water weight 18. Is Amu equal to g mol. Pu1amu pu16605E-24 g Now divide one gram by one mole. 12 amuatom 12 gmol. One Mole is the molecular weight expressed as grams - so a mole of Carbon 12 is BY DEFINITION exactly 12 grams.
 Source: slidetodoc.com
Source: slidetodoc.com
Converting Daltons to gmol One Dalton is one AMU atomic Mass Unit. One Mole is the molecular weight expressed as grams - so a mole of Carbon 12 is BY DEFINITION exactly 12 grams. Thus the molar mass of magnesium is 243050 gmol compared to carbons molar mass of 12011 gmol. For a further explanation visit. Although mass can be expressed as both amu and gmol gmol is the most useful system of units for laboratory chemistry.
 Source: slideplayer.com
Source: slideplayer.com
On the next page though it appears to say that 4115 amu4115 gmol. Is Amu equal to g mol. This property simplifies many chemical computations. The atomic mass is useful in chemistry when it is paired with the mole concept. Every single molecule of water is made up of 2 Hydrogen and 1 Oxygen Atom.
 Source: slideplayer.com
Source: slideplayer.com
015 atomic mass units so one MOLE of water weight 18. The atomic mass of an element measured in amu is the same as the mass in grams of one mole of an element. The mass of a single atom of an element amu is numerically equal to the mass g of 1 mol of that element regardless of the element. 12 amuatom 12 gmol. Similarly one may ask what is the difference between atomic.
 Source: youtube.com
Source: youtube.com
The atomic mass of an element measured in amu is the same as the mass in grams of one mole of an element. The mass in grams of one mole of substance is called molar mass. The atomic mass is useful in chemistry when it is paired with the mole concept. Pu1g mol-1 fracpu1 gpu6022E23 atom pu16605E-24 g atom-1 Its the same number. One atom of sulfur has a mass of 3207 amu.
 Source: slideplayer.com
Source: slideplayer.com
The atomic mass of an element measured in amu is the same as the mass in grams of one mole of an element. To check this look at the mass of an amu when converted to grams. Because different atoms and molecules have different weights to convert from AMU to mole you must know exactly the particle youre using in order to know the weight of one mole of it. Atomic Mass Unit to Gram Conversion Table How to Convert Atomic Mass Unit to Gram 1 u 16605402E-24 g. Although mass can be expressed as both amu and gmol gmol is the most useful system of units for laboratory chemistry.
 Source: slideplayer.com
Source: slideplayer.com
To check this look at the mass of an amu when converted to grams. This property simplifies many chemical computations. Another property of Avogadros Number is that the mass of one MOLE of substance is equal to that substances molecular weight. Convert the AMU value to grams by multiplying it by 167 x 10-24. The atomic mass of an element measured in amu is the same as the mass in grams of one mole of an element.
 Source: slidetodoc.com
Source: slidetodoc.com
Pu1g mol-1 pu 1. The atomic mass of an element measured in amu is the same as the mass in grams of one mole of an element. To check this look at the mass of an amu when converted to grams. The mass of one mole of atoms of a pure element in grams is equivalent to the atomic mass of that element in atomic mass units amu or in grams per mole gmol. Note that rounding errors may occur so always check the results.
 Source: pediaa.com
Source: pediaa.com
Number of particles in one mole of any substance is equal to avogadro constant N60231023. One AMU is 112th of the weight of a Carbon 12 Atom. The atomic mass of an element measured in amu is the same as the mass in grams of one mole of an element. 1 amuatom 1 gmol. The mass of one mole of atoms of a pure element in grams is equivalent to the atomic mass of that element in atomic mass units amu or in grams per mole gmol.
 Source: slideplayer.com
Source: slideplayer.com
Why is Amu equal to g mol. 1 mole is equal to 1 moles 7 AmU or 48802891 grams. Its somewhat convenient the relation between grams to amu that is in the sense that the value in amu of a particular atomcompound is pretty much the same with the mass of a mole of that atomcompound. Although mass can be expressed as both amu and g mol g mol is the most useful system of units for laboratory chemistry. One atomic mass unit amu is approximately the mass of one nucleon either a single proton or neutron and is numerically equivalent to 1 gmol.
 Source: slideplayer.com
Source: slideplayer.com
So mass of one nucleon 1 amu 1N1660510-24 grams. The mass of one mole of atoms of a pure element in grams is equivalent to the atomic mass of that element in atomic mass units amu or in grams per mole gmol. For example mean molecular weight of water is 18. For a further explanation visit. On the next page though it appears to say that 4115 amu4115 gmol.
 Source: slideplayer.com
Source: slideplayer.com
Another property of Avogadros Number is that the mass of one MOLE of substance is equal to that substances molecular weight. Atomic Mass Unit to Gram Conversion Table How to Convert Atomic Mass Unit to Gram 1 u 16605402E-24 g. Gram is used in our day to day life to express the mass of goods that we use whereas amu is used for minute scale measurements. Pu1g mol-1 pu 1. This number has been determined through experimentation to be 6022 1023 and is known as Avogadros number.
 Source: getchemistryhelp.com
Source: getchemistryhelp.com
The mass of an atom in amu is numerically the same as the mass of one mole of atoms of the element in grams. The atomic mass is useful in chemistry when it is paired with the mole concept. This number has been determined through experimentation to be 6022 1023 and is known as Avogadros number. Atomic Mass Unit to Gram Conversion Table How to Convert Atomic Mass Unit to Gram 1 u 16605402E-24 g. Type in your own numbers in the form to convert the units.
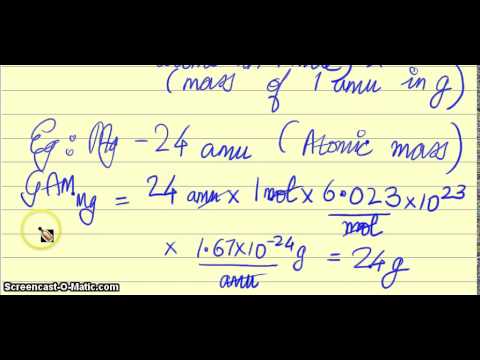 Source: youtube.com
Source: youtube.com
For example mean molecular weight of water is 18. Converting Daltons to gmol One Dalton is one AMU atomic Mass Unit. For example mean molecular weight of water is 18. Although mass can be expressed as both amu and g mol g mol is the most useful system of units for laboratory chemistry. Another property of Avogadros Number is that the mass of one MOLE of substance is equal to that substances molecular weight.
 Source: slidetodoc.com
Source: slidetodoc.com
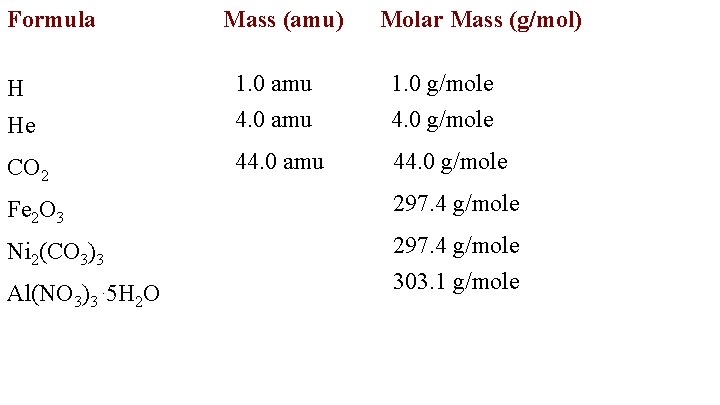
The mass of one mole of atoms of a pure element in grams is equivalent to the atomic mass of that element in atomic mass units amu or in grams per mole gmol. This number has been determined through experimentation to be 6022 1023 and is known as Avogadros number. One atomic mass unit amu is approximately the mass of one nucleon either a single proton or neutron and is numerically equivalent to 1 gmol. Pu1g mol-1 pu 1. Gram is used in our day to day life to express the mass of goods that we use whereas amu is used for minute scale measurements.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Thus the molar mass of magnesium is 243050 gmol compared to carbons molar mass of 12011 gmol. Now lets get back to your question. Where U Uranium. One AMU is 112th of the weight of a Carbon 12 Atom. The mass of a single atom of an element amu is numerically equal to the mass g of 1 mol of that element regardless of the element.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title is grams per mol the same as amu by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






