Your Is atomic weight grams per mole images are ready in this website. Is atomic weight grams per mole are a topic that is being searched for and liked by netizens now. You can Download the Is atomic weight grams per mole files here. Download all royalty-free photos and vectors.
If you’re searching for is atomic weight grams per mole images information linked to the is atomic weight grams per mole keyword, you have pay a visit to the ideal blog. Our site always gives you hints for downloading the maximum quality video and picture content, please kindly hunt and find more informative video articles and images that fit your interests.
Is Atomic Weight Grams Per Mole. This is due to the fact that the number of atoms in a mole is chosen in such a way for this to happen. In other words never let your molar mass be the measured value that determines how. Dec 02 2016 Moles let you straight learn weight from the periodic desk eg. 1 ounce is 116 of a pound and about 2835 grams.
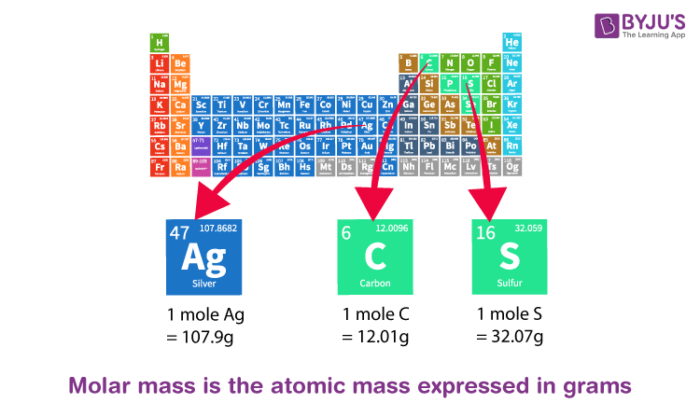 What Is Atomic Mass List Of Elements Sorted By Atomic Mass Of Iron Sulphur Potassium Chlorine Etc From byjus.com
What Is Atomic Mass List Of Elements Sorted By Atomic Mass Of Iron Sulphur Potassium Chlorine Etc From byjus.com
Molar mass units Gram per mole gmol Kilogram per mole kgmol Standard atomic weight Hydrogen H Oxygen O Sulfur S Chlorine Cl Iron Fe Molecular mass Hydrogen molecule H2 Water molecule. The definition of atomic mass the mole and molar mass are all directly or indirectly related to carbon-12. 115 rows The atomic mass is useful in chemistry when it is paired with the mole concept. The atomic mass of carbon would be 1201 grams per mole of carbon atoms. This is defined as 0001 kilogram per mole or 1 gram per mole. Thus since the atomic mass of iron is 55847 amu one mole of iron atoms would weigh 55847 grams.
The mass of one mole of carbon-12 atoms is exactly 12 grams.
This leads to two important facts. And this is exactly equal to 16 gmol or 16 kgkmol. Ounces are often used in cooking especially with ingredients in small quantities. The first table of standard atomic weight atomic mass was published by John Dalton 17661844 in 1805 based on a system in which the relative atomic mass of hydrogen was defined as 1. While atomic mass is measured in amu molar mass is measured in grams per mole. The formula weight is simply the weight in atomic mass units of.
 Source: br.pinterest.com
Source: br.pinterest.com
Molar mass massmole gmol. Ounces are often used in cooking especially with ingredients in small quantities. Gram per mole gmol - Molar mass units molar mass. Well to figure that out and thats why this periodic table of elements is useful we just have to figure out the molar mass of the constituent elements. The atomic mass of carbon would be 1201 grams per mole of carbon atoms.
 Source: x-engineer.org
Source: x-engineer.org
Numerically its the same as the elements atomic mass in units of amu atomic mass units. The corresponding amount-specific mass is MO 16 Daent. 1 stone is about 635 kilograms or exactly 14 pounds. Molar mass units Gram per mole gmol Kilogram per mole kgmol Standard atomic weight Hydrogen H Oxygen O Sulfur S Chlorine Cl Iron Fe Molecular mass Hydrogen molecule H2 Water molecule. Finally we add up the weights of all the atoms to get the total molecular weight of.
 Source: pinterest.com
Source: pinterest.com
Dec 02 2016 Moles let you straight learn weight from the periodic desk eg. These relative atomic masses were based on the stoichiometric. The definition of atomic mass the mole and molar mass are all directly or indirectly related to carbon-12. Thus since the atomic mass of iron is 55847 amu one mole of iron atoms would weigh 55847 grams. 1Molar mass is the mass of one mole per single element while atomic mass is the mass of an atom at rest or is the number of protons and neutrons.

The formula weight is simply the weight in atomic mass units of. The first table of standard atomic weight atomic mass was published by John Dalton 17661844 in 1805 based on a system in which the relative atomic mass of hydrogen was defined as 1. The atomic mass of carbon would be 1201 grams per mole of carbon atoms. In other words 1 gram per mole is 2 times smaller than a hydrogen h - standard atomic weight. How many moles are in 1 gram.
 Source: pinterest.com
Source: pinterest.com
The atomic mass of carbon would be 1201 grams per mole of carbon atoms. The formula weight is simply the weight in atomic mass units of. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by 100. Dec 02 2016 Moles let you straight learn weight from the periodic desk eg. In other words never let your molar mass be the measured value that determines how.
 Source: pinterest.com
Source: pinterest.com
5988 kg 5988 g. 115 rows The atomic mass is useful in chemistry when it is paired with the mole concept. Its easier to work with grams so convert the mass. As you already know how the grams to moles conversion work find the number of moles. The formula weight is simply the weight in atomic mass units of.
 Source: pinterest.com
Source: pinterest.com
This converts atomic units to grams per mole making the molar mass of hydrogen 1007 grams per mole of. 115 rows The atomic mass is useful in chemistry when it is paired with the mole concept. ArO maODa 16. M 6 l 998 kgm³ 0006 m³ 998 kgm³ 5988 kg. The formula weight is simply the weight in atomic mass units of.
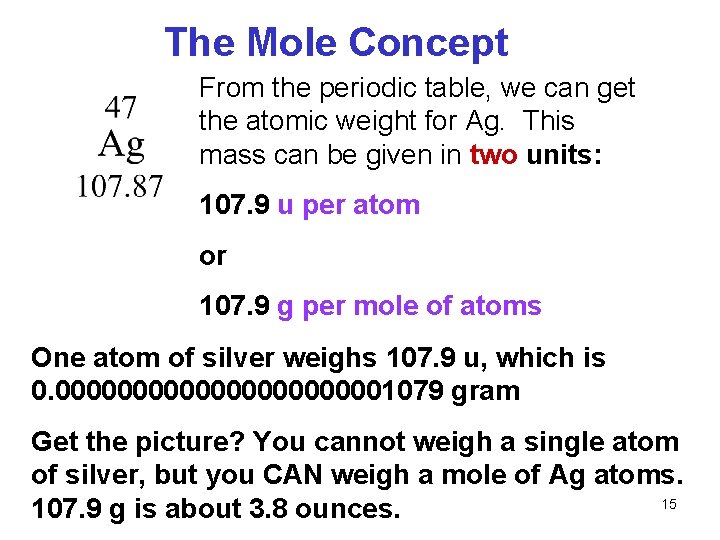 Source: slidetodoc.com
Source: slidetodoc.com
Our carbon atom has 6 protons 6 neutrons 12. Numerically its the same as the elements atomic mass in units of amu atomic mass units. Finally we add up the weights of all the atoms to get the total molecular weight of. The definition of atomic mass the mole and molar mass are all directly or indirectly related to carbon-12. So if we first look at carbon carbon we see from this periodic table of elements has a molar mass of 1201 grams per mole.
 Source: slidetodoc.com
Source: slidetodoc.com
Gram per mole gmol - Molar mass units molar mass. 5988 kg 5988 g. This is defined as 0001 kilogram per mole or 1 gram per mole. And this is exactly equal to 16 gmol or 16 kgkmol. Ounces are often used in cooking especially with ingredients in small quantities.
 Source: slideplayer.com
Source: slideplayer.com
One mole of any atom has a weight equal to the atomic mass expressed in grams. The mass of one mole of carbon-12 atoms is exactly 12 grams. M 6 l 998 kgm³ 0006 m³ 998 kgm³ 5988 kg. So if we first look at carbon carbon we see from this periodic table of elements has a molar mass of 1201 grams per mole. The atomic mass of carbon would be 1201 grams per mole of carbon atoms.
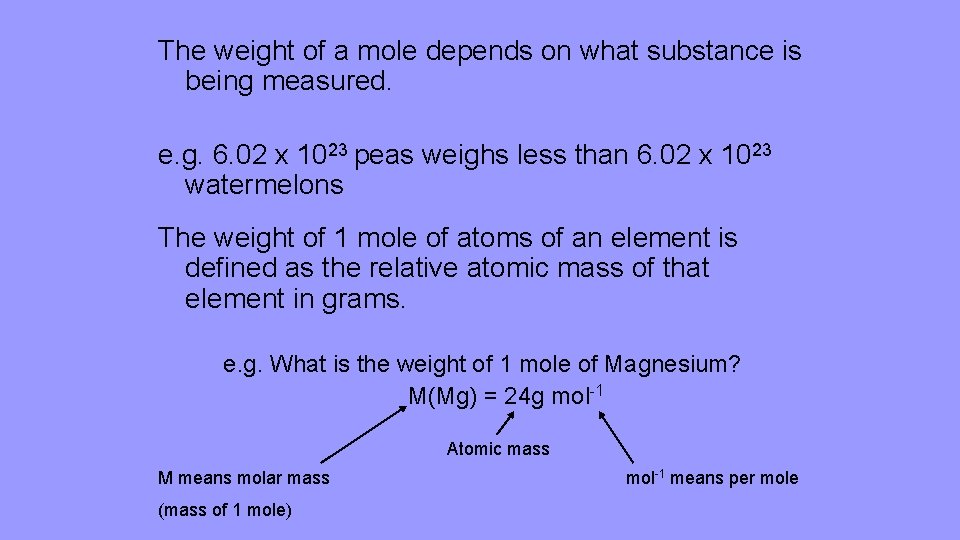 Source: slidetodoc.com
Source: slidetodoc.com
The atomic weight of a component is the burden in grams of 1 mole of atoms of that aspect. The mass of one mole of carbon-12 atoms is exactly 12 grams. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by 100. While atomic mass is measured in amu molar mass is measured in grams per mole. Our carbon atom has 6 protons 6 neutrons 12.
 Source: byjus.com
Source: byjus.com
HOWEVER make sure that you use at least as many significant figures in your molar mass as the measurement with the fewest significant figures. The first table of standard atomic weight atomic mass was published by John Dalton 17661844 in 1805 based on a system in which the relative atomic mass of hydrogen was defined as 1. While atomic mass is measured in amu molar mass is measured in grams per mole. The weight of one mole is 84 grams so the weight of 7 grams is. One mole of any atom has a weight equal to the atomic mass expressed in grams.
 Source: pinterest.com
Source: pinterest.com
The definition of atomic mass the mole and molar mass are all directly or indirectly related to carbon-12. Since the unified atomic mass unit symbol. Tables of atomic weights list the numerical values of atomic-scale masses in daltons–eg. 115 rows The atomic mass is useful in chemistry when it is paired with the mole concept. The mass of one atom of carbon-12 the atomic mass of carbon-12 is exactly 12 atomic mass units.
 Source: wikihow.com
Source: wikihow.com
Dec 02 2016 Moles let you straight learn weight from the periodic desk eg. 1 ounce is 116 of a pound and about 2835 grams. Ounces are often used in cooking especially with ingredients in small quantities. In other words never let your molar mass be the measured value that determines how. These relative atomic masses were based on the stoichiometric.
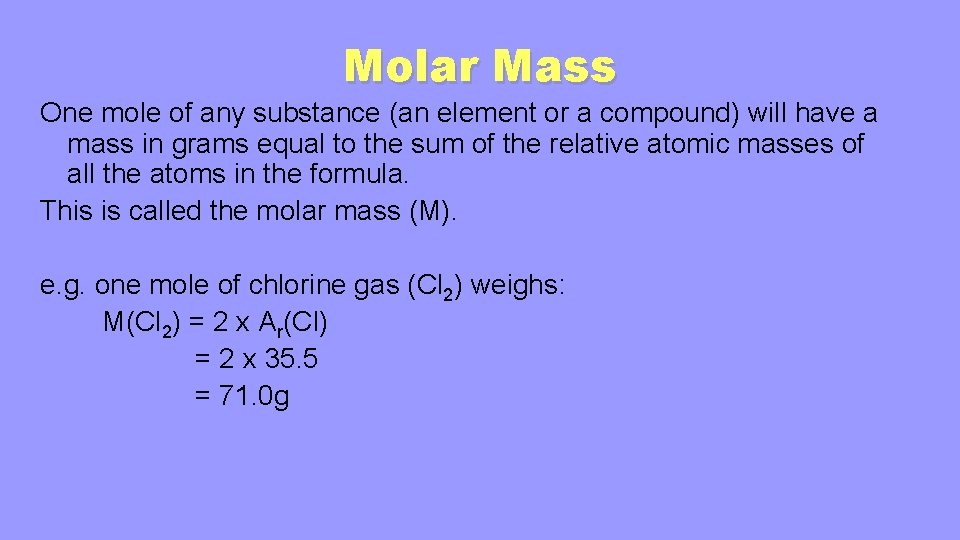 Source: slidetodoc.com
Source: slidetodoc.com
1 mole of N₂ is 28 g or 1 mole of NaCl is 585 g. The atomic mass of carbon would be 1201 grams per mole of carbon atoms. Therefore the mole is a unit for that physical quantity. This converts atomic units to grams per mole making the molar mass of hydrogen 1007 grams per mole of. The atomic mass of hydrogen is about one gram per mole.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title is atomic weight grams per mole by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






