Your In one mole of c6h14o6 how many grams of oxygen images are ready. In one mole of c6h14o6 how many grams of oxygen are a topic that is being searched for and liked by netizens now. You can Find and Download the In one mole of c6h14o6 how many grams of oxygen files here. Get all royalty-free vectors.
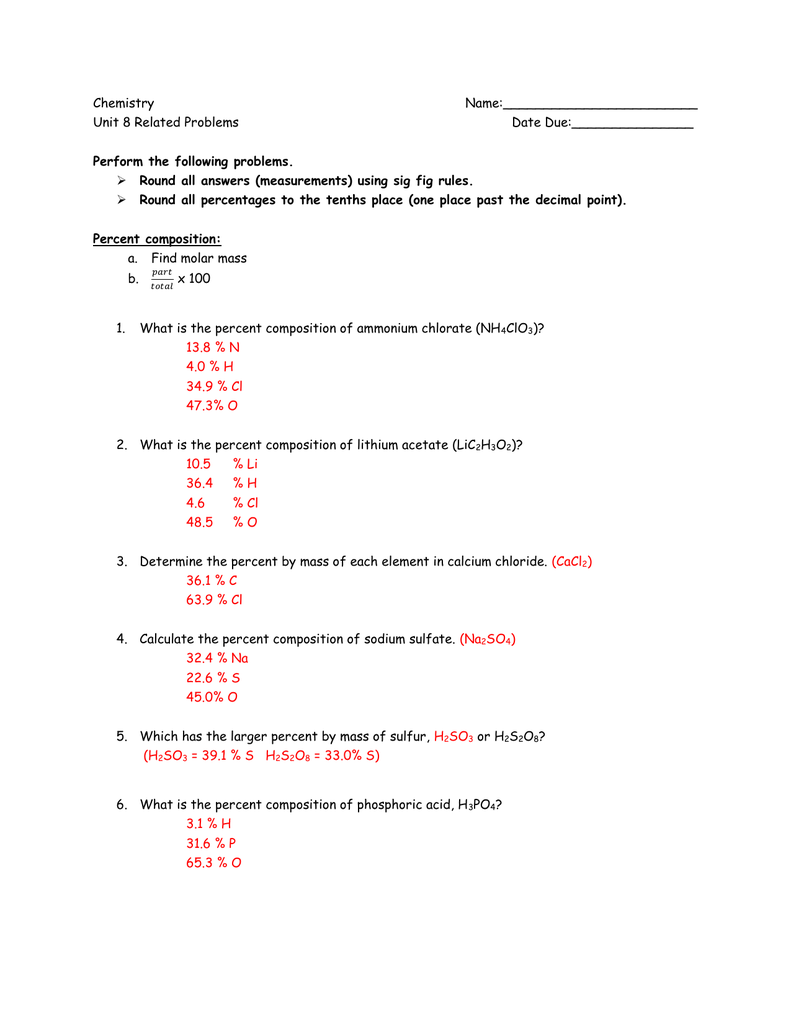
If you’re looking for in one mole of c6h14o6 how many grams of oxygen pictures information linked to the in one mole of c6h14o6 how many grams of oxygen interest, you have pay a visit to the right site. Our site frequently provides you with hints for seeking the maximum quality video and picture content, please kindly hunt and locate more informative video content and graphics that fit your interests.
In One Mole Of C6h14o6 How Many Grams Of Oxygen. Now in mol of CO2 there are 602210 23 molecules and in each molecule there are 2 atoms of O. Carbon hydrogen and oxygen. How many moles of C6H12O6 are formed when 0250 mole of CO2 is consumed in the reaction 6 CO2 6 H2O C6H12O6 6 O2. The SI base unit for amount of substance is the mole.
 Mole Practice Worksheet 4 Stoichiometry Scientific Notation Word Problems Practices Worksheets Scientific Notation Practice From pinterest.com
Mole Practice Worksheet 4 Stoichiometry Scientific Notation Word Problems Practices Worksheets Scientific Notation Practice From pinterest.com
2360 moles of lead II oxide. 050 moles of calcium nitrate. 1 mol H3PO4 4 mol O 1 mol H3PO4 16 g 1 mol O 64 g O 98 g H3PO4 x 100 653 P. The unbalanced equation for this reaction is as follows. How many elements are in C6 H12 o6. 0031 moles of aluminium iodide.
Now in mol of CO2 there are 602210 23 molecules and in each molecule there are 2 atoms of O.
Molecular weight of Glucose or grams The molecular formula for Glucose is C6H12O6. 250L x 1 mol224 L 0112 moles CO2. Glucose C6H12O6 reacts with oxygen in the body according to the following balanced equation. This will give you the number of moles of that substance that are in the specified mass. Sugar is made up of carbon hydrogen and oxygen atoms. 0436 moles of ammonium chloride.

Carbon hydrogen and oxygen. Chemistry questions and answers. Now in mol of CO2 there are 602210 23 molecules and in each molecule there are 2 atoms of O. Do a quick conversion. See also what human body system could be fossilized.
 Source: pinterest.com
Source: pinterest.com
See also what human body system could be fossilized. 1 mole is equal to 1 moles Glucose or 18015588 grams. How many grams of oxygen are needed to produce 100 mole of water. The SI base unit for amount of substance is the mole. From the balanced equation 4NH3 7O2 - 4NO2 6H2O.
 Source: studylib.net
Source: studylib.net
A 66 b 96 c 180 d 36 x 1023 e none of these values 33 The formula for the most stable oxide of titanium is TiO2. Now in mol of CO2 there are 602210 23 molecules and in each molecule there are 2 atoms of O. This number is referred to as Avogadros constant 6022 E23. 1 mole is equal to 1 moles Nitrogen or 140067 grams. When 1 mole of glucose C6H12O6 is completely burned into CO2 how many moles of H2O are produced.
 Source: pinterest.com
Source: pinterest.com
Molar mass of oxygen O16gmol1. A 14 b 26 c 14 x 1023 d 84 x 1023 e none of these values 22 In one mole of C6H14O6 there are approximately how many grams of oxygen. A 66 b 96 c 180 d 36 x 1023 e none of these values 33 The formula for the most stable oxide of titanium is TiO2. When 1 mole of glucose C6H12O6 is completely burned into CO2 how many moles of H2O are produced. 1 mole is equal to 1 moles Glucose or 18015588 grams.
 Source: studylib.net
Source: studylib.net
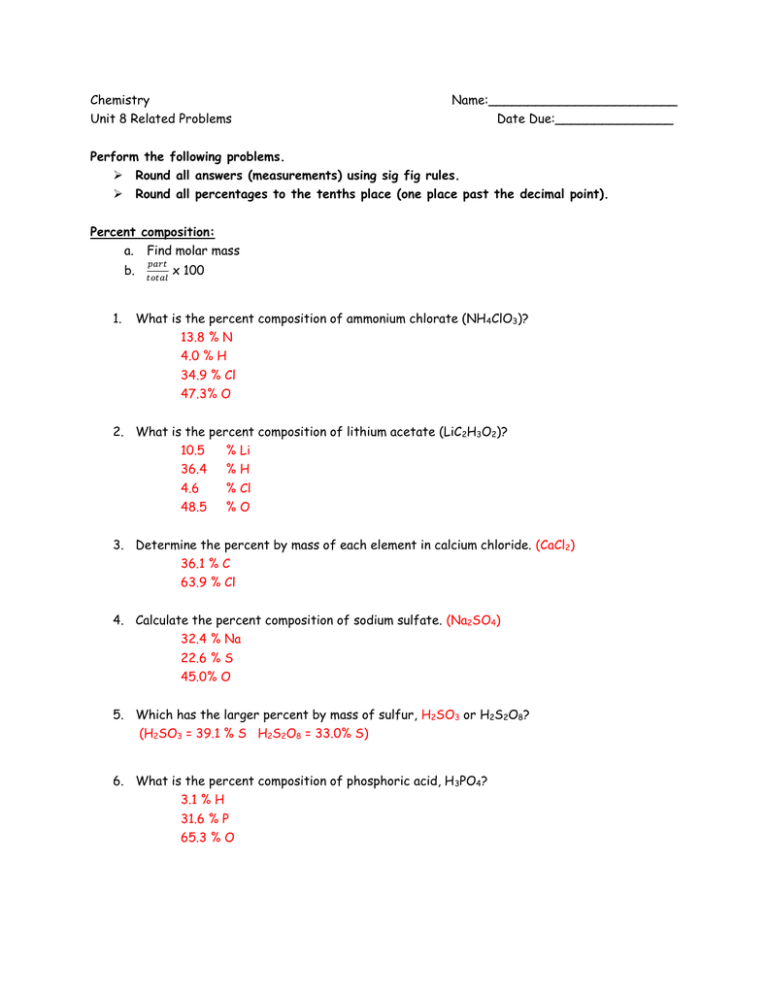
Note that rounding errors may occur so always check the results. Convert the following number of moles of chemical into its corresponding mass in grams. How many moles of C6H12O6 are formed when 0250 mole of CO2 is consumed in the reaction 6 CO2 6 H2O C6H12O6 6 O2. To find the molar mass we need to add the masses of individual elements which constitute one glucose molecule. How many grams of.
 Source: studylib.net
Source: studylib.net
Now in mol of CO2 there are 602210 23 molecules and in each molecule there are 2 atoms of O. 1 mol H3PO4 4 mol O 1 mol H3PO4 16 g 1 mol O 64 g O 98 g H3PO4 x 100 653 P. You can view more details on each measurement unit. This number is referred to as Avogadros constant 6022 E23. From the balanced equation 4NH3 7O2 - 4NO2 6H2O.
 Source: slideplayer.com
Source: slideplayer.com
1077 moles of magnesium phosphate. For the oxidation of glucose C6H12O6 602 6H20 6CO2 how many grams of oxygen gas will be consumed when 950 g of glucose is. Mass of 1 mole of glucose C6H12O6 6 1201 12 101 6 1600 g 18018 g using atomic weight data to 2 decimals 1 mole of carbon atoms weighs 1201 g and there are 6 moles of C atoms in 1 mole of glucose so the mass of carbon. Molar mass of oxygen O16gmol1. Molar mass of Carbon C12gmol1.
 Source: pinterest.com
Source: pinterest.com
Sugar is made up of carbon hydrogen and oxygen atoms. For the oxidation of glucose C6H12O6 602 6H20 6CO2 how many grams of oxygen gas will be consumed when 950 g of glucose is. The unbalanced equation for this reaction is as follows. Sugar is made up of carbon hydrogen and oxygen atoms. 1 grams C6H12O6 00055507486072617 mole using the molecular weight calculator and the molar mass of C6H12O6.
 Source: studylib.net
Source: studylib.net
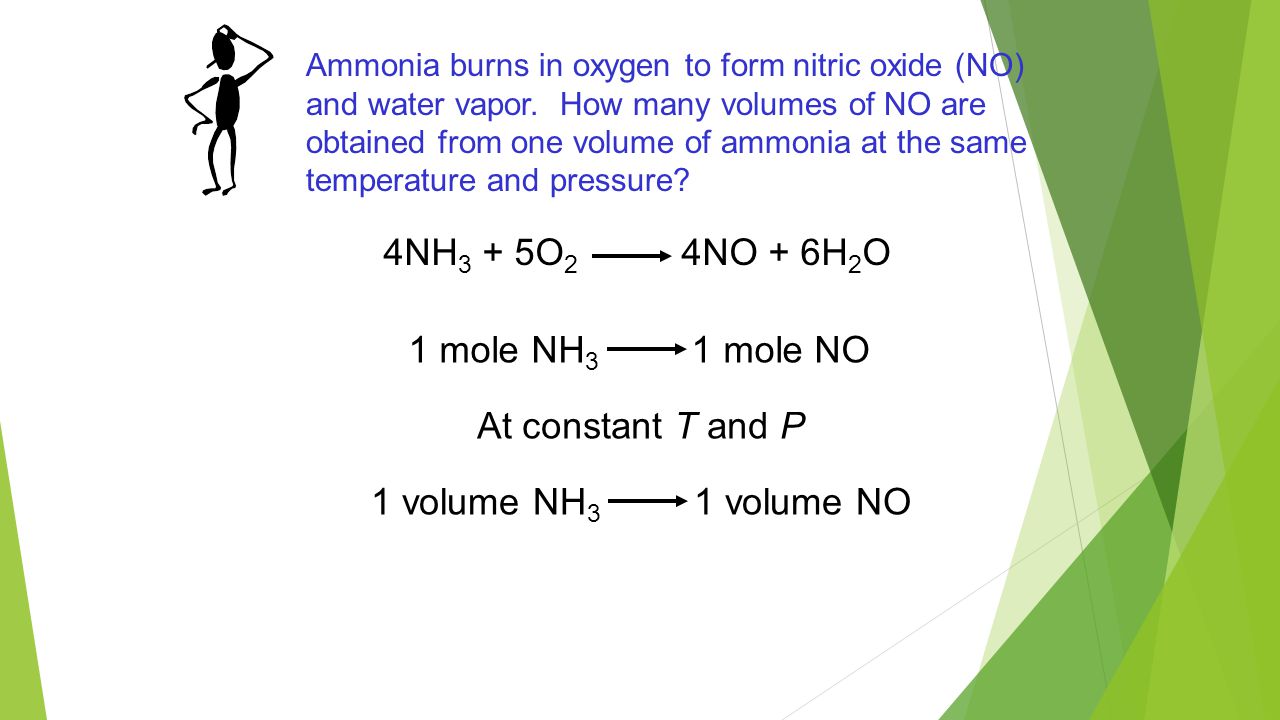
Moles B moles of A g B grams B moles A 1 mol B 1. 1472 grams C6H12O6 1 mole C6H12O6180156 grams6 moles O1 mole C6H12O6160 grams1 mole O 784 grams oxygen —– How many atoms of carbon are in 711g of glucose. Do a quick conversion. One mole of oxygen and one mole of hydrogen both contain the same number of atoms. Chemistry questions and answers.

One mole of oxygen and one mole of hydrogen both contain the same number of atoms. C6H12O6 602 6 CO2 6 H2O I a. Chemistry questions and answers. This compound is also known as Glucose or Fructose or. You can view more details on each measurement unit.
 Source: studylib.net
Source: studylib.net
4 rows Molar mass of C6H12O6 18015588 gmol. C6H12O6 602 6 CO2 6 H2O I a. How many moles of C6H12O6 are formed when 0250 mole of CO2 is consumed in the reaction 6 CO2 6 H2O C6H12O6 6 O2. How many grams is C6H4Cl2. Molar mass of Carbon C12gmol1.
 Source: pinterest.com
Source: pinterest.com
From the periodic table 6 carbons 12011 12 hydrogens 1008 6 oxygens 15999 180156 gmol Step 2. Molar mass of Carbon C12gmol1. Molar mass of Hydrogen H1gmol1. Now we know that. Molar mass of oxygen O16gmol1.
 Source: pinterest.com
Source: pinterest.com
Now we know that. 1472 grams C6H12O6 1 mole C6H12O6180156 grams6 moles O1 mole C6H12O6160 grams1 mole O 784 grams oxygen —– How many atoms of carbon are in 711g of glucose. 1 grams C6H12O6 00055507486072617 mole using the molecular weight calculator and the molar mass of C6H12O6. Now we know that. How many grams is C6H4Cl2.
 Source: studylib.net
Source: studylib.net
Mass of 1 mole of glucose C6H12O6 6 1201 12 101 6 1600 g 18018 g using atomic weight data to 2 decimals 1 mole of carbon atoms weighs 1201 g and there are 6 moles of C atoms in 1 mole of glucose so the mass of carbon in 1 mole of glucose 6 1201 g 7206 g. How many atoms are bound together to form a glucose molecule C6H12O6. Chemistry questions and answers. For the oxidation of glucose C6H12O6 602 6H20 6CO2 how many grams of oxygen gas will be consumed when 950 g of glucose is. 1 mole is equal to 1 moles Nitrogen or 140067 grams.
 Source: pinterest.com
Source: pinterest.com
Sugar is made up of carbon hydrogen and oxygen atoms. Glucose C6H12O6 reacts with oxygen in the body according to the following balanced equation. The unbalanced equation for this reaction is as follows. How many moles of C6H12O6 are formed when 0250 mole of CO2 is consumed in the reaction 6 CO2 6 H2O C6H12O6 6 O2. How many elements are in C6 H12 o6.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title in one mole of c6h14o6 how many grams of oxygen by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






